Vaccine strategies for the Mtb/HIV copandemic
- PMID: 33083030
- PMCID: PMC7555484
- DOI: 10.1038/s41541-020-00245-9
Vaccine strategies for the Mtb/HIV copandemic
Abstract
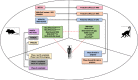
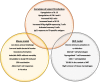
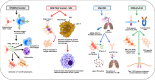
One-third of world's population is predicted to be infected with tuberculosis (TB). The resurgence of this deadly disease has been inflamed by comorbidity with human immunodeficiency virus (HIV). The risk of TB in people living with HIV (PLWH) is 15-22 times higher than people without HIV. Development of a single vaccine to combat both diseases is an ardent but tenable ambition. Studies have focused on the induction of specific humoral and cellular immune responses against HIV-1 following recombinant BCG (rBCG) expressing HIV-1 antigens. Recent advances in the TB vaccines led to the development of promising candidates such as MTBVAC, the BCG revaccination approach, H4:IC31, H56:IC31, M72/AS01 and more recently, intravenous (IV) BCG. Modification of these vaccine candidates against TB/HIV coinfection could reveal key correlates of protection in a representative animal model. This review discusses the (i) potential TB vaccine candidates that can be exploited for use as a dual vaccine against TB/HIV copandemic (ii) progress made in the realm of TB/HIV dual vaccine candidates in small animal model, NHP model, and human clinical trials (iii) the failures and promising targets for a successful vaccine strategy while delineating the correlates of vaccine-induced protection.
Keywords: Diseases; Immunology; Microbiology; Pathogenesis.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
Similar articles
-
A phase 1b randomized study of the safety and immunological responses to vaccination with H4:IC31, H56:IC31, and BCG revaccination in Mycobacterium tuberculosis-uninfected adolescents in Cape Town, South Africa.EClinicalMedicine. 2020 Mar 18;21:100313. doi: 10.1016/j.eclinm.2020.100313. eCollection 2020 Apr. EClinicalMedicine. 2020. PMID: 32382714 Free PMC article.
-
Safety and efficacy of tuberculosis vaccine candidates in low- and middle-income countries: a systematic review of randomised controlled clinical trials.BMC Infect Dis. 2023 Feb 24;23(1):120. doi: 10.1186/s12879-023-08092-4. BMC Infect Dis. 2023. PMID: 36829123 Free PMC article.
-
A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates.PLoS Pathog. 2019 Mar 4;15(3):e1007643. doi: 10.1371/journal.ppat.1007643. eCollection 2019 Mar. PLoS Pathog. 2019. PMID: 30830940 Free PMC article.
-
Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination.N Engl J Med. 2018 Jul 12;379(2):138-149. doi: 10.1056/NEJMoa1714021. N Engl J Med. 2018. PMID: 29996082 Free PMC article. Clinical Trial.
-
Recombinant BCG: Innovations on an Old Vaccine. Scope of BCG Strains and Strategies to Improve Long-Lasting Memory.Front Immunol. 2014 Apr 7;5:152. doi: 10.3389/fimmu.2014.00152. eCollection 2014. Front Immunol. 2014. PMID: 24778634 Free PMC article. Review.
Cited by
-
Vaccines against Tuberculosis: Where Are We Now?Vaccines (Basel). 2023 May 22;11(5):1013. doi: 10.3390/vaccines11051013. Vaccines (Basel). 2023. PMID: 37243117 Free PMC article. Review.
-
Enhanced Glycosylation Caused by Overexpression of Rv1002c in a Recombinant BCG Promotes Immune Response and Protects against Mycobacterium tuberculosis Infection.Vaccines (Basel). 2024 Jun 4;12(6):622. doi: 10.3390/vaccines12060622. Vaccines (Basel). 2024. PMID: 38932351 Free PMC article.
-
Microbiomes Detected by Bronchoalveolar Lavage Fluid Metagenomic Next-Generation Sequencing among HIV-Infected and Uninfected Patients with Pulmonary Infection.Microbiol Spectr. 2023 Aug 17;11(4):e0000523. doi: 10.1128/spectrum.00005-23. Epub 2023 Jul 12. Microbiol Spectr. 2023. PMID: 37436163 Free PMC article.
-
Recombinant BCG Expressing IL-12 as A Novel Immunomodulatory Strategy for Allergic Asthma: Opportunities and Challenges.Clin Rev Allergy Immunol. 2025 Jun 7;68(1):54. doi: 10.1007/s12016-025-09066-x. Clin Rev Allergy Immunol. 2025. PMID: 40483388 Review.
-
Bridging the gaps to overcome major hurdles in the development of next-generation tuberculosis vaccines.Front Immunol. 2023 Aug 11;14:1193058. doi: 10.3389/fimmu.2023.1193058. eCollection 2023. Front Immunol. 2023. PMID: 37638056 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

