Double Retinal Tamponade for Treatment of Rhegmatogenous Retinal Detachment with Proliferative Vitreoretinopathy and Inferior Breaks
- PMID: 33083050
- PMCID: PMC7563057
- DOI: 10.1155/2020/6938627
Double Retinal Tamponade for Treatment of Rhegmatogenous Retinal Detachment with Proliferative Vitreoretinopathy and Inferior Breaks
Abstract
Purpose: To evaluate the efficacy and safety of the simultaneous use of short-term perfluoro-n-octane (PFO) with perfluoropropane (C3F8) gas to achieve retinal reattachment in eyes with rhegmatogenous retinal detachment (RRD) with proliferative vitreoretinopathy (PVR) grade C and multiple retinal breaks including inferior breaks.
Design: This is a prospective interventional case series study. Patients and Methods. The study was a prospective noncomparative interventional study. It included 30 eyes of 30 patients who had RRD with PVR grade C and multiple retinal breaks including inferior tears attending the vitreoretinal unit of Minia University Hospital, Egypt. The mean age was 50.2 ± 10.63 years; 18 patients were females and 12 were males. Combined phacoemulsification and 23 G pars plana vitrectomy (PPV) with double retinal tamponade by C3F8 and PFO were done, and PFO was removed in 10-14 days. The patients were followed up for one year. The primary outcome was to achieve successful retinal reattachment, and the secondary outcomes were visual improvement and occurrence of complications.
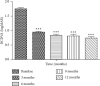
Results: Successful retinal reattachment was obtained in 28 eyes out of 30 (93.3%), and 2 eyes (6.7%) had recurrent RD. Best-corrected distance visual acuity (BCDVA) in logMAR was significantly improved from baseline 1.74 ± 0.05 to 0.93 ± 0.04, 0.82 ± 0.05, 0.80 ± 0.07, and 0.73 ± 0.055 at follow-up visits 3, 6, and 9 months and one year, respectively (P ≤ 0.001). There were no serious ocular complications recorded.
Conclusions: The results of this study indicated that primary vitrectomy with simultaneous use of both C3F8 and short-term PFO as retinal tamponades was effective and safe in the management of complex cases of RRD with PVR grade C and inferior breaks. This trial is registered with NCT04168255.
Copyright © 2020 Mohamed Farouk Abdelkader et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest or financial interests; in addition, these data have not been published before.
References
-
- Heussen N., Feltgen N., Walter P., et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment study (SPR Study): predictive factors for functional outcome. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2011;249:1129–1136. doi: 10.1007/s00417-011-1619-7. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous