LIM domain proteins Pinch1/2 regulate chondrogenesis and bone mass in mice
- PMID: 33083097
- PMCID: PMC7553939
- DOI: 10.1038/s41413-020-00108-y
LIM domain proteins Pinch1/2 regulate chondrogenesis and bone mass in mice
Abstract
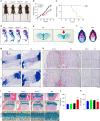
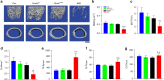
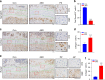
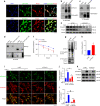
The LIM domain-containing proteins Pinch1/2 regulate integrin activation and cell-extracellular matrix interaction and adhesion. Here, we report that deleting Pinch1 in limb mesenchymal stem cells (MSCs) and Pinch2 globally (double knockout; dKO) in mice causes severe chondrodysplasia, while single mutant mice do not display marked defects. Pinch deletion decreases chondrocyte proliferation, accelerates cell differentiation and disrupts column formation. Pinch loss drastically reduces Smad2/3 protein expression in proliferative zone (PZ) chondrocytes and increases Runx2 and Col10a1 expression in both PZ and hypertrophic zone (HZ) chondrocytes. Pinch loss increases sclerostin and Rankl expression in HZ chondrocytes, reduces bone formation, and increases bone resorption, leading to low bone mass. In vitro studies revealed that Pinch1 and Smad2/3 colocalize in the nuclei of chondrocytes. Through its C-terminal region, Pinch1 interacts with Smad2/3 proteins. Pinch loss increases Smad2/3 ubiquitination and degradation in primary bone marrow stromal cells (BMSCs). Pinch loss reduces TGF-β-induced Smad2/3 phosphorylation and nuclear localization in primary BMSCs. Interestingly, compared to those from single mutant mice, BMSCs from dKO mice express dramatically lower protein levels of β-catenin and Yap1/Taz and display reduced osteogenic but increased adipogenic differentiation capacity. Finally, ablating Pinch1 in chondrocytes and Pinch2 globally causes severe osteopenia with subtle limb shortening. Collectively, our findings demonstrate critical roles for Pinch1/2 and a functional redundancy of both factors in the control of chondrogenesis and bone mass through distinct mechanisms.
Keywords: Bone; Bone quality and biomechanics.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures

Similar articles
-
Focal adhesion proteins Pinch1 and Pinch2 regulate bone homeostasis in mice.JCI Insight. 2019 Nov 14;4(22):e131692. doi: 10.1172/jci.insight.131692. JCI Insight. 2019. PMID: 31723057 Free PMC article.
-
Brief research report: Effects of Pinch deficiency on cartilage homeostasis in adult mice.Front Cell Dev Biol. 2023 Jan 19;11:1116128. doi: 10.3389/fcell.2023.1116128. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36743414 Free PMC article.
-
Consequences of loss of PINCH2 expression in mice.J Cell Sci. 2005 Dec 15;118(Pt 24):5899-910. doi: 10.1242/jcs.02686. Epub 2005 Nov 29. J Cell Sci. 2005. PMID: 16317048
-
The bone-liver interaction modulates immune and hematopoietic function through Pinch-Cxcl12-Mbl2 pathway.Cell Death Differ. 2024 Jan;31(1):90-105. doi: 10.1038/s41418-023-01243-9. Epub 2023 Dec 7. Cell Death Differ. 2024. PMID: 38062244 Free PMC article.
-
Regulation of bone development and extracellular matrix protein genes by RUNX2.Cell Tissue Res. 2010 Jan;339(1):189-95. doi: 10.1007/s00441-009-0832-8. Epub 2009 Aug 1. Cell Tissue Res. 2010. PMID: 19649655 Review.
Cited by
-
Kindlin-2 inhibits TNF/NF-κB-Caspase 8 pathway in hepatocytes to maintain liver development and function.Elife. 2023 Jan 9;12:e81792. doi: 10.7554/eLife.81792. Elife. 2023. PMID: 36622102 Free PMC article.
-
Targeting Piezo1 channel to alleviate intervertebral disc degeneration.J Orthop Translat. 2025 Mar 8;51:145-158. doi: 10.1016/j.jot.2025.01.006. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 40129609 Free PMC article.
-
Loss of Pinch Proteins Causes Severe Degenerative Disc Disease-Like Lesions in Mice.Aging Dis. 2023 Oct 1;14(5):1818-1833. doi: 10.14336/AD.2023.0212. Aging Dis. 2023. PMID: 37196110 Free PMC article.
-
Targeting miR-29 mitigates skeletal senescence and bolsters therapeutic potential of mesenchymal stromal cells.Cell Rep Med. 2024 Aug 20;5(8):101665. doi: 10.1016/j.xcrm.2024.101665. Cell Rep Med. 2024. PMID: 39168101 Free PMC article.
-
Osteocyte β1 integrin loss causes low bone mass and impairs bone mechanotransduction in mice.J Orthop Translat. 2022 May 17;34:60-72. doi: 10.1016/j.jot.2022.03.008. eCollection 2022 May. J Orthop Translat. 2022. PMID: 35615639 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

