A Comparison of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance in a Group at High Risk of HCC Development
- PMID: 33083277
- PMCID: PMC7548851
- DOI: 10.1159/000506834
A Comparison of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance in a Group at High Risk of HCC Development
Abstract
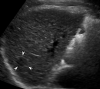
Background and aims: Biannual ultrasonography (US) is a current recommendation for hepatocellular carcinoma (HCC) surveillance in a high-risk group. The sensitivity of US, however, has been low in patients with a high risk of developing HCC. We aimed to compare sensitivity for HCC of biannual US and two-phase low-dose computed tomography (LDCT) in patients with a high risk of HCC.
Methods: In this prospective single-arm study, participants with an annual risk of HCC greater than 5% (based on a risk index of ≥2.33) and who did not have a history of HCC were enrolled from November 2014 to July 2016. Participants underwent paired biannual US and two-phase LDCT 1-3 times. Two-phase LDCT included arterial and 3-min delayed phases. The sensitivity, specificity, and positive predictive value of HCC detection using US and two-phase LDCT were compared using a composite algorithm as a standard of reference.
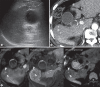
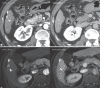
Results: Of the 139 enrolled participants, 137 underwent both the biannual US and two-phase LDCT at least once and had follow-up images. Among them, 27 cases of HCC (mean size: 14 ± 4 mm) developed in 24 participants over 1.5 years. Two-phase LDCT showed a significantly higher sensitivity (83.3% [20/24] vs. 29.2% [7/24], p < 0.001) and specificity (95.6% [108/113] vs. 87.7% [99/113], p =0.03) than US. A false-positive result was reported in 14 participants at US and 5 participants at two-phase LDCT, resulting in a significantly higher positive predictive value of two-phase LDCT (33.3% [7/21] vs. 80% [20/25], p < 0.001).
Conclusions: Patients with a risk index ≥2.33 showed a high annual incidence of HCC development in our study, and two-phase LDCT showed significantly higher sensitivity and specificity for HCC detection than US.
Keywords: Cirrhosis; Hepatocellular carcinoma; Low-dose CT; Surveillance; Ultrasonography.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
Jeong Hee Yoon: activities related to the present article: disclosed no relevant relationship. Activities not related to the present article: the author previously received grants from Bayer; and personal fees from Philips Healthcare, Samsung Electronics, and Bayer. Jeong Min Lee: activities related to the present article: disclosed no relevant relationship. Activities not related to the present article: the author previously received nonfinancial technical support from Siemens Healthineers and Philips Healthcare; grants from Dongseo Medical, CMS, Acuzen, Starmed, RF Medical, and Bayer; and personal fees from Bayer Healthcare for lectures. Ijin Joo, Dong Ho Lee, Su Joa Ahn, Ju Hyun Jeon, Seung-taak Kim, Eun Ju Cho, Su Jong Yu, and Yoon Jun Kim: disclosed no relevant relationship. Jeong-Hoon Lee: activities related to the present article: disclosed no relevant relationship. Activities not related to the present article: the author previously received lecture fees from GreenCross Cell, Daewoong Pharmaceuticals, and Gilead Korea. Jung-Hwan Yoon: activities related to the present article: disclosed no relevant relationship. Activities not related to the present article: the author previously received research grants from Bayer, Bukwang Pharmaceuticals, and Daewoong Pharmaceuticals.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68((6)):394–424. - PubMed
-
- Kim BH, Lim YS, Kim EY, Kong HJ, Won YJ, Han S, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018 Feb;33((2)):475–83. - PubMed
-
- Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017 Aug;67((2)):302–9. - PubMed
LinkOut - more resources
Full Text Sources

