Safety, pharmacokinetics, and pharmacodynamics of Gln-1062, a prodrug of galantamine
- PMID: 33083515
- PMCID: PMC7551138
- DOI: 10.1002/trc2.12093
Safety, pharmacokinetics, and pharmacodynamics of Gln-1062, a prodrug of galantamine
Abstract
Introduction: Gln-1062 (MEMOGAIN) is an intranasally administered lipophilic prodrug of galantamine. Based on high brain-to-blood concentrations observed in pre-clinical studies, Gln-1062 is expected to have superior cognitive efficacy compared to oral galantamine.
Methods: Forty-eight healthy elderly subjects were randomized 12:4 to Gln-1062 (5.5, 11, or 22 mg, b.i.d., for 7 days) or placebo. Safety, tolerability, pharmacokinetics, and pharmacodynamics were assessed repeatedly. Pharmacokinetics were compared with 16 mg oral galantamine.
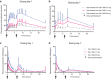
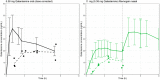
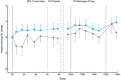
Results: Gln-1062 up to 22 mg, b.i.d., was well tolerated. Gln-1062 plasma concentrations increased immediately following dosing (median Tmax of 0.5 hour [range 0.5-1.0]). Cmax and AUC0-last increased in a dose-linear manner over all three dose levels. Gln-1062 was rapidly cleaved into galantamine. Gln-1062 significantly improved adaptive tracking (sustained attention) with 1.95% (95% confidence interval [CI] 0.630-3.279, P = 0.0055) compared to placebo after correction for individual baseline performance.
Discussion: Gln-1062 was considered to be safe and caused fewer gastrointestinal side effects than oral galantamine. Gln-1062 behaved pharmacokinetically as expected and improved performance on cognitive tests.
Keywords: Alzheimer's disease; cholinesterase inhibitor; dementia; galantamine; pharmacodynamics; pharmacokinetics; pharmacology; randomized controlled trial; safety; side effects.
© 2020 The Authors. Alzheimer's & Dementia: Translational Research & Clinical Interventions published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
This study was funded by Neurodyn Life Sciences Inc., now known as Alpha Cognition Inc. D.G. Kay is employee of Alpha Cognition Inc. All other authors report no conflicts of interest.
Figures
References
-
- Maelicke A, Hoeffle‐Maas A, Ludwig J, et al. Memogain is a galantamine pro‐drug having dramatically reduced adverse effects and enhanced efficacy. J Mol Neurosci. 2010;40(1‐2):135‐137. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
