The genomic landscape of metastatic histologic special types of invasive breast cancer
- PMID: 33083532
- PMCID: PMC7560857
- DOI: 10.1038/s41523-020-00195-4
The genomic landscape of metastatic histologic special types of invasive breast cancer
Abstract
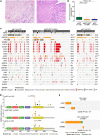
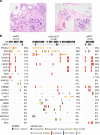
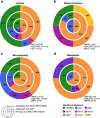
Histologic special types of breast cancer (BC) account for ~20% of BCs. Large sequencing studies of metastatic BC have focused on invasive ductal carcinomas of no special type (IDC-NSTs). We sought to define the repertoire of somatic genetic alterations of metastatic histologic special types of BC. We reanalyzed targeted capture sequencing data of 309 special types of BC, including metastatic and primary invasive lobular carcinomas (ILCs; n = 132 and n = 127, respectively), mixed mucinous (n = 5 metastatic and n = 14 primary), micropapillary (n = 12 metastatic and n = 8 primary), and metaplastic BCs (n = 6 metastatic and n = 5 primary), and compared metastatic histologic special types of BC to metastatic IDC-NSTs matched according to clinicopathologic characteristics and to primary special type BCs. The genomic profiles of metastatic and primary special types of BC were similar. Important differences, however, were noted: metastatic ILCs harbored a higher frequency of genetic alterations in TP53, ESR1, FAT1, RFWD2, and NF1 than primary ILCs, and in CDH1, PIK3CA, ERBB2, TBX3, NCOR1, and RFWD2 than metastatic IDC-NSTs. Metastatic ILCs displayed a higher mutational burden, and more frequently dominant APOBEC mutational signatures than primary ILCs and matched metastatic IDC-NSTs. ESR1 and NCOR mutations were frequently detected in metastatic mixed mucinous BCs, whereas PIK3CA and TP53 were the most frequently altered genes in metastatic micropapillary and metaplastic BCs, respectively. Taken together, primary and metastatic BCs histologic special types have remarkably similar repertoires of somatic genetic alterations. Metastatic ILCs more frequently harbor APOBEC mutational signatures than primary ILCs and metastatic IDC-NSTs.
Keywords: Breast cancer.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsJ.S.R.-F. reports receiving personal/consultancy fees from Goldman Sachs and REPARE Therapeutics, membership of the scientific advisory boards of VolitionRx and Page.AI, and ad hoc membership of the scientific advisory boards of Roche Tissue Diagnostics, Ventana Medical Systems, Novartis, Genentech and InVicro, outside the scope of this study. P.R. reports consulting/advisory board for Novartis and institutional research support from Illumina and GRAIL, Inc. S.C. has received research support from Daichi Sankyo and consulting fees from Novartis, Sermonix, BMS, Context Therapeutics, Revolution Medicine, Paige AI, and Eli Lilly. G.Z. has received research support from ThermoFisher Scientific, AstraZeneka UK, and SIDRA Medicine. All other authors declare no competing interests.
Figures


References
-
- WHO Classification of Tumors Editorial Board. Breast tumours. WHO Classification of Tumors 5th edn (IARC, Lyon, 2019).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

