Policy, toxicology and physicochemical considerations on the inhalation of high concentrations of food flavour
- PMID: 33083547
- PMCID: PMC7541606
- DOI: 10.1038/s41538-020-00075-y
Policy, toxicology and physicochemical considerations on the inhalation of high concentrations of food flavour
Abstract
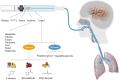
Food flavour ingredients are required by law to obtain prior approval from regulatory bodies, such as the U.S. Food and Drug Administration (FDA) or the European Food Safety Authority (EFSA) in terms of toxicological data and intended use levels. However, there are no regulations for labelling the type and concentration of flavour additives on the product, primarily due to their low concentration in food and generally recognised as safe (GRAS) status determined by the flavour and extract manufacturers' association (FEMA). Their status for use in e-cigarettes and other vaping products challenges these fundamental assumptions, because their concentration can be over ten-thousand times higher than in food, and the method of administration is through inhalation, which is currently not evaluated by the FEMA expert panel. This work provides a review of some common flavour ingredients used in food and vaping products, their product concentrations, inhalation toxicity and aroma interactions reported with different biological substrates. We have identified several studies, which suggest that the high concentrations of flavour through inhalation may pose a serious health threat, especially in terms of their cytotoxicity. As a result of the wide range of possible protein-aroma interactions reported in our diet and metabolism, including links to several non-communicable diseases, we suggest that it is instrumental to update current flavour- labelling regulations, and support new strategies of understanding the effects of flavour uptake on the digestive and respiratory systems, in order to prevent the onset of future non-communicable diseases.
Keywords: Chemical safety; Drug regulation; Environmental impact; Toxicology.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures


References
-
- Parker, J. K., Elmore, S. & Methven, L. Flavour Development, Analysis and Perception in Food and Beverages. (Elsevier, 2014).
-
- Fisk, I. D. 6—Aroma release. in Flavour Development, Analysis and Perception in Food and Beverages. (Woodhead Publishing, 2015). 10.1016/B978-1-78242-103-0.00006-0.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

