Digital Measures That Matter to Patients: A Framework to Guide the Selection and Development of Digital Measures of Health
- PMID: 33083687
- PMCID: PMC7548919
- DOI: 10.1159/000509725
Digital Measures That Matter to Patients: A Framework to Guide the Selection and Development of Digital Measures of Health
Abstract
Background: With the rise of connected sensor technologies, there are seemingly endless possibilities for new ways to measure health. These technologies offer researchers and clinicians opportunities to go beyond brief snapshots of data captured by traditional in-clinic assessments, to redefine health and disease. Given the myriad opportunities for measurement, how do research or clinical teams know what they should be measuring? Patient engagement, early and often, is paramount to thoughtfully selecting what is most important. Regulators encourage stakeholders to have a patient focus but actionable steps for continuous engagement are not well defined. Without patient-focused measurement, stakeholders risk entrenching digital versions of poor traditional assessments and proliferating low-value tools that are ineffective, burdensome, and reduce both quality and efficiency in clinical care and research.
Summary: This article synthesizes and defines a sequential framework of core principles for selecting and developing measurements in research and clinical care that are meaningful for patients. We propose next steps to drive forward the science of high-quality patient engagement in support of measures of health that matter in the era of digital medicine.
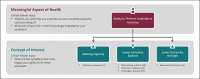
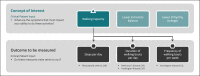
Key messages: All measures of health should be meaningful, regardless of the product's regulatory classification, type of measure, or context of use. To evaluate meaningfulness of signals derived from digital sensors, the following four-level framework is useful: Meaningful Aspect of Health, Concept of Interest, Outcome to be measured, and Endpoint (exclusive to research). Incorporating patient input is a dynamic process that requires more than a single, transactional touch point but rather should be conducted continuously throughout the measurement selection process. We recommend that developers, clinicians, and researchers reevaluate processes for more continuous patient engagement in the development, deployment, and interpretation of digital measures of health.
Keywords: Digital measures; Digital medicine; Patient engagement.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no disclosures to report.
Figures


References
-
- FDA-NIH Biomarker Working Group BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet] Silver Spring (MD): Food and Drug Administration (US); 2016. Glossary. 2016 Jan;28 [Updated 2020 Mar 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338448/ - PubMed
-
- FDA CDER Patient-Focused Drug Development. 2020 Apr;21 Available in: https://www.fda.gov/drugs/development-approval-process-drugs/cder-patien....
-
- Chapter 57. In. Kraus WE. Heart Failure: A Companion to Braunwald's Heart Disease. Exercise in Heart Failure; 2011. pp. 834–44.
-
- Schoindre Y, Meune C, Dinh-Xuan AT, Avouac J, Kahan A, Allanore Y. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J Rheumatol. 2009 Jul;36((7)):1481–5. Available in: https://www.ncbi.nlm.nih.gov/pubmed/19487260. - PubMed
-
- CTTI Use Cases for Developing Novel Endpoints Generated Using Mobile Technology: Duchenne Muscular Dystrophy. 2020. Mar, Available in: https://www.ctti-clinicaltrials.org/files/usecase-duchenne.pdf.
Publication types
LinkOut - more resources
Full Text Sources

