Metal- and UV- Catalyzed Oxidation Results in Trapped Amyloid-β Intermediates Revealing that Self-Assembly Is Required for Aβ-Induced Cytotoxicity
- PMID: 33083713
- PMCID: PMC7516296
- DOI: 10.1016/j.isci.2020.101537
Metal- and UV- Catalyzed Oxidation Results in Trapped Amyloid-β Intermediates Revealing that Self-Assembly Is Required for Aβ-Induced Cytotoxicity
Abstract
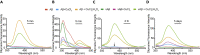
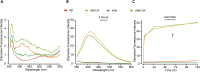
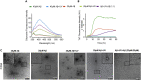
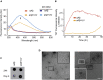
Dityrosine (DiY), via the cross-linking of tyrosine residues, is a marker of protein oxidation, which increases with aging. Amyloid-β (Aβ) forms DiY in vitro and DiY-cross-linked Aβ is found in the brains of patients with Alzheimer disease. Metal- or UV- catalyzed oxidation of Aβ42 results in an increase in DiY cross-links. Using DiY as a marker of oxidation, we compare the self-assembly propensity and DiY cross-link formation for a non-assembly competent variant of Aβ42 (vAβ) with wild-type Aβ42. Oxidation results in the formation of trapped wild-type Aβ assemblies with increased DiY cross-links that are unable to elongate further. Assembly-incompetent vAβ and trapped Aβ assemblies are non-toxic to neuroblastoma cells at all stages of self-assembly, in contrast to oligomeric, non-cross-linked Aβ. These findings point to a mechanism of toxicity that necessitates dynamic self-assembly whereby trapped Aβ assemblies and assembly-incompetent variant Aβ are unable to result in cell death.
Keywords: Biochemical Mechanism; Molecular Neuroscience; Neurotoxicology.
© 2020 The Author(s).
Conflict of interest statement
The authors declare there are no competing interests.
Figures




References
-
- Ali F.E., Leung A., Cherny R.A., Mavros C., Barnham K.J., Separovic F., Barrow C.J. Dimerisation of N-acetyl-L-tyrosine ethyl ester and Abeta peptides via formation of dityrosine. Free Radic. Res. 2006;40:1–9. - PubMed
-
- Ali F.E., Separovic F., Barrow C.J., Cherny R.A., Fraser F., Bush A.I., Masters C.L., Barnham K.J. Methionine regulates copper/hydrogen peroxide oxidation products of Aβ. J. Pept. Sci. 2005;11:353–360. - PubMed
-
- Atwood C.S., Perry G., Zeng H., Kato Y., Jones W.D., Ling K.Q., Huang X., Moir R.D., Wang D., Sayre L.M. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-beta. Biochemistry. 2004;43:560–568. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

