Exome Sequencing Implicates Impaired GABA Signaling and Neuronal Ion Transport in Trigeminal Neuralgia
- PMID: 33083721
- PMCID: PMC7554653
- DOI: 10.1016/j.isci.2020.101552
Exome Sequencing Implicates Impaired GABA Signaling and Neuronal Ion Transport in Trigeminal Neuralgia
Abstract
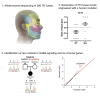
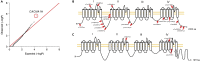
Trigeminal neuralgia (TN) is a common, debilitating neuropathic face pain syndrome often resistant to therapy. The familial clustering of TN cases suggests that genetic factors play a role in disease pathogenesis. However, no unbiased, large-scale genomic study of TN has been performed to date. Analysis of 290 whole exome-sequenced TN probands, including 20 multiplex kindreds and 70 parent-offspring trios, revealed enrichment of rare, damaging variants in GABA receptor-binding genes in cases. Mice engineered with a TN-associated de novo mutation (p.Cys188Trp) in the GABAA receptor Cl- channel γ-1 subunit (GABRG1) exhibited trigeminal mechanical allodynia and face pain behavior. Other TN probands harbored rare damaging variants in Na+ and Ca+ channels, including a significant variant burden in the α-1H subunit of the voltage-gated Ca2+ channel Cav3.2 (CACNA1H). These results provide exome-level insight into TN and implicate genetically encoded impairment of GABA signaling and neuronal ion transport in TN pathogenesis.
Keywords: Genomics; Neuroscience; Structural Biology.
© 2020 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Trigeminal neuralgia and genetics: A systematic review.Mol Pain. 2021 Jan-Dec;17:17448069211016139. doi: 10.1177/17448069211016139. Mol Pain. 2021. PMID: 34000891 Free PMC article.
-
Electrophysiological and computational analysis of Cav3.2 channel variants associated with familial trigeminal neuralgia.Mol Brain. 2022 Nov 17;15(1):91. doi: 10.1186/s13041-022-00978-9. Mol Brain. 2022. PMID: 36397158 Free PMC article.
-
Mechanistic contribution of CaV3.2 calcium channels to trigeminal neuralgia pathophysiology not clarified.F1000Res. 2022 Jun 29;11:718. doi: 10.12688/f1000research.122997.2. eCollection 2022. F1000Res. 2022. PMID: 36128556 Free PMC article.
-
Familial trigeminal neuralgia - a systematic clinical study with a genomic screen of the neuronal electrogenisome.Cephalalgia. 2020 Jul;40(8):767-777. doi: 10.1177/0333102419897623. Epub 2020 Jan 13. Cephalalgia. 2020. PMID: 31928344 Free PMC article.
-
Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain.Mol Pain. 2011 Jan 10;7:5. doi: 10.1186/1744-8069-7-5. Mol Pain. 2011. PMID: 21219657 Free PMC article. Review.
Cited by
-
Intranasal CRMP2-Ubc9 inhibitor regulates Na V 1.7 to alleviate trigeminal neuropathic pain.Pain. 2024 Mar 1;165(3):573-588. doi: 10.1097/j.pain.0000000000003053. Epub 2023 Sep 26. Pain. 2024. PMID: 37751532 Free PMC article.
-
Trigeminal neuralgia and genetics: A systematic review.Mol Pain. 2021 Jan-Dec;17:17448069211016139. doi: 10.1177/17448069211016139. Mol Pain. 2021. PMID: 34000891 Free PMC article.
-
[Additional experience with medicinal treatment of trigeminal nerve pain].Schmerz. 2024 Oct;38(5):359-361. doi: 10.1007/s00482-024-00820-2. Epub 2024 Aug 14. Schmerz. 2024. PMID: 39141098 German. No abstract available.
-
Electrophysiological and computational analysis of Cav3.2 channel variants associated with familial trigeminal neuralgia.Mol Brain. 2022 Nov 17;15(1):91. doi: 10.1186/s13041-022-00978-9. Mol Brain. 2022. PMID: 36397158 Free PMC article.
-
Trigeminal neuralgia.Nat Rev Dis Primers. 2024 May 30;10(1):39. doi: 10.1038/s41572-024-00523-z. Nat Rev Dis Primers. 2024. PMID: 38816415 Review.
References
-
- Ackerman M.J., Siu B.L., Sturner W.Q., Tester D.J., Valdivia C.R., Makielski J.C., Towbin J.A. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. - PubMed
-
- Ahmed O.L., Akinyele O.A., Akindayo A.O., Bamidele K. Management of trigeminal neuralgia using amitriptyline and pregablin combination therapy. Afr. J. Biomed. Res. 2012;15:201–203.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

