Mitochondrial Superoxide Dismutase Specifies Early Neural Commitment by Modulating Mitochondrial Dynamics
- PMID: 33083732
- PMCID: PMC7522125
- DOI: 10.1016/j.isci.2020.101564
Mitochondrial Superoxide Dismutase Specifies Early Neural Commitment by Modulating Mitochondrial Dynamics
Abstract
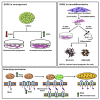
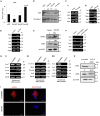
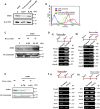
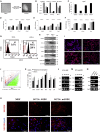
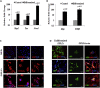
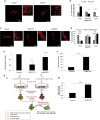
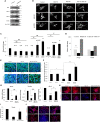
Studies revealing molecular mechanisms underlying neural specification have majorly focused on the role played by different transcription factors, but less on non-nuclear components. Earlier, we reported mitochondrial superoxide dismutase (SOD2) to be essential for self-renewal and pluripotency of mouse embryonic stem cells (mESCs). In the present study, we found SOD2 to be specifically required for neural lineage, but not the meso- or endoderm specification. Temporally, SOD2 regulated early neural genes, but not the matured genes, by modulating mitochondrial dynamics-specifically by enhancing the mitochondrial fusion protein Mitofusin 2 (MFN2). Bio-complementation strategy further confirmed SOD2 to enhance mitochondrial fusion process independent of its antioxidant activity. Over-expression of SOD2 along with OCT4, but neither alone, transdifferentiated mouse fibroblasts to neural progenitor-like colonies, conclusively proving the neurogenic potential of SOD2. In conclusion, our findings accredit a novel role for SOD2 in early neural lineage specification.
Keywords: Developmental Genetics; Developmental Neuroscience; Molecular Genetics.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Manganese Superoxide Dismutase Gene Expression Is Induced by Nanog and Oct4, Essential Pluripotent Stem Cells' Transcription Factors.PLoS One. 2015 Dec 7;10(12):e0144336. doi: 10.1371/journal.pone.0144336. eCollection 2015. PLoS One. 2015. PMID: 26642061 Free PMC article.
-
Novel role of mitochondrial manganese superoxide dismutase in STAT3 dependent pluripotency of mouse embryonic stem cells.Sci Rep. 2015 Mar 30;5:9516. doi: 10.1038/srep09516. Sci Rep. 2015. PMID: 25822711 Free PMC article.
-
Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension.Arterioscler Thromb Vasc Biol. 2019 Aug;39(8):1682-1698. doi: 10.1161/ATVBAHA.119.312613. Epub 2019 Jun 13. Arterioscler Thromb Vasc Biol. 2019. PMID: 31189433
-
Acquired Mitochondrial Abnormalities, Including Epigenetic Inhibition of Superoxide Dismutase 2, in Pulmonary Hypertension and Cancer: Therapeutic Implications.Adv Exp Med Biol. 2016;903:29-53. doi: 10.1007/978-1-4899-7678-9_3. Adv Exp Med Biol. 2016. PMID: 27343087 Review.
-
Insights into the Dichotomous Regulation of SOD2 in Cancer.Antioxidants (Basel). 2017 Nov 3;6(4):86. doi: 10.3390/antiox6040086. Antioxidants (Basel). 2017. PMID: 29099803 Free PMC article. Review.
Cited by
-
Dose-Dependent Effect of Melatonin on BAT Thermogenesis in Zücker Diabetic Fatty Rat: Future Clinical Implications for Obesity.Antioxidants (Basel). 2022 Aug 25;11(9):1646. doi: 10.3390/antiox11091646. Antioxidants (Basel). 2022. PMID: 36139720 Free PMC article.
-
Imaging Structural and Functional Dynamics in Xenopus Neurons.Cold Spring Harb Protoc. 2022 Feb 1;2022(2):pdb.top106773. doi: 10.1101/pdb.top106773. Cold Spring Harb Protoc. 2022. PMID: 34531329 Free PMC article.
-
Dose-Dependent Response to the Environmental Pollutant Dichlorodipheniletylhene (DDE) in HepG2 Cells: Focus on Cell Viability and Mitochondrial Fusion/Fission Proteins.Toxics. 2021 Oct 20;9(11):270. doi: 10.3390/toxics9110270. Toxics. 2021. PMID: 34822661 Free PMC article.
-
Gastroprotective Effects of Ganoderma lucidum Polysaccharides with Different Molecular Weights on Ethanol-Induced Acute Gastric Injury in Rats.Nutrients. 2022 Apr 1;14(7):1476. doi: 10.3390/nu14071476. Nutrients. 2022. PMID: 35406089 Free PMC article.
References
-
- Arrázola M.S., Andraini T., Szelechowski M., Mouledous L., Arnauné-Pelloquin L., Davezac N., Belenguer P., Rampon C., Miquel M.C. Mitochondria in developmental and adult neurogenesis. Neurotox. Res. 2019;36:257–267. - PubMed
-
- Beckervordersandforth R., Ebert B., Schäffner I., Moss J., Fiebig C., Shin J., Moore D.L., Ghosh L., Trinchero M.F., Stockburger C. Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron. 2017;93:560–573.e6. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

