Drosophila hedgehog can act as a morphogen in the absence of regulated Ci processing
- PMID: 33084577
- PMCID: PMC7679133
- DOI: 10.7554/eLife.61083
Drosophila hedgehog can act as a morphogen in the absence of regulated Ci processing
Abstract
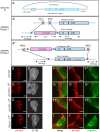
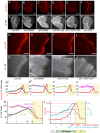
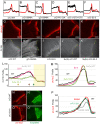
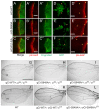
Extracellular Hedgehog (Hh) proteins induce transcriptional changes in target cells by inhibiting the proteolytic processing of full-length Drosophila Ci or mammalian Gli proteins to nuclear transcriptional repressors and by activating the full-length Ci or Gli proteins. We used Ci variants expressed at physiological levels to investigate the contributions of these mechanisms to dose-dependent Hh signaling in Drosophila wing imaginal discs. Ci variants that cannot be processed supported a normal pattern of graded target gene activation and the development of adults with normal wing morphology, when supplemented by constitutive Ci repressor, showing that Hh can signal normally in the absence of regulated processing. The processing-resistant Ci variants were also significantly activated in the absence of Hh by elimination of Cos2, likely acting through binding the CORD domain of Ci, or PKA, revealing separate inhibitory roles of these two components in addition to their well-established roles in promoting Ci processing.
Keywords: Ci; D. melanogaster; Protein Kinase A; cell biology; costal 2; developmental biology; hedgehog; morphogen; signaling.
Plain language summary
Morphogens play a crucial role in determining how cells are organized in developing organisms. These chemical signals act over a wide area, and the amount of signal each cell receives typically initiates a sequence of events that spatially pattern the multiple cells of an organ or tissue. One of the most well-studied groups of morphogens are the hedgehog proteins, which are involved in the development of many animals, ranging from flies to humans. In fruit flies, hedgehog proteins kickstart a cascade of molecular changes that switch on a set of 'target' genes. They do this by ultimately altering the activity of a protein called cubitus interruptus, which comes in two lengths: a long version called Ci-155 and a short version called Ci-75. When hedgehog is absent, Ci-155 is kept in an inactive state in the cytoplasm, where it is slowly converted into its shorter form, Ci-75: this repressor protein is then able to access the nucleus, where it switches ‘off’ the target genes. However, when a hedgehog signal is present, the processing of Ci into its shorter form is inhibited. Instead, Ci-155 becomes activated by a separate mechanism that allows the long form protein to enter the nucleus and switch ‘on’ the target genes. But it was unclear whether hedgehog requires both of these mechanisms in order to act as a morphogen and regulate the activity of developmental genes. To answer this question, Little et al. mutated the gene for Ci in the embryo of fruit flies, so that the Ci-155 protein could no longer be processed into Ci-75. Examining the developing wings of these flies revealed that the genes targeted by hedgehog are still activated in the correct pattern. In some parts of the wing, Ci-75 is required to switch off specific sets of genes. But when Little et al. blocked these genes, by adding a gene that constantly produces the Ci repressor in the presence or absence of hedgehog, the adult flies still developed normally structured wings. This suggests that hedgehog does not need to regulate the processing of Ci-155 into Ci-75 in order to perform its developmental role. Previous work showed that when one of the major mechanisms used by hedgehog to activate Ci-155 is blocked, fruit flies are still able to develop normal wings. Taken together with the findings of Little et al., this suggests that the two mechanisms induced by hedgehog can compensate for each other, and independently regulate the development of the fruit fly wing. These mechanisms, which are also found in humans, have been linked to birth defects and several common types of cancer, and understanding how they work could help the development of new treatments.
© 2020, Little et al.
Conflict of interest statement
JL, EG, AS, DK No competing interests declared
Figures






References
-
- Blair SS. Engrailed expression in the anterior lineage compartment of the developing wing blade of Drosophila. Development. 1992;115:21–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

