Covalent labeling of nucleic acids
- PMID: 33084688
- PMCID: PMC7116832
- DOI: 10.1039/d0cs00600a
Covalent labeling of nucleic acids
Abstract
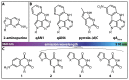
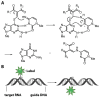
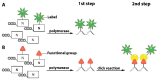
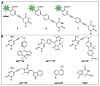
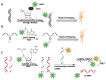
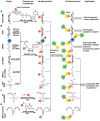
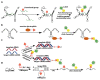
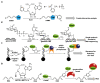
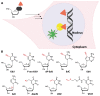
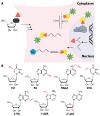
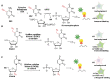
Labeling of nucleic acids is required for many studies aiming to elucidate their functions and dynamics in vitro and in cells. Out of the numerous labeling concepts that have been devised, covalent labeling provides the most stable linkage, an unrivaled choice of small and highly fluorescent labels and - thanks to recent advances in click chemistry - an incredible versatility. Depending on the approach, site-, sequence- and cell-specificity can be achieved. DNA and RNA labeling are rapidly developing fields that bring together multiple areas of research: on the one hand, synthetic and biophysical chemists develop new fluorescent labels and isomorphic nucleobases as well as faster and more selective bioorthogonal reactions. On the other hand, the number of enzymes that can be harnessed for post-synthetic and site-specific labeling of nucleic acids has increased significantly. Together with protein engineering and genetic manipulation of cells, intracellular and cell-specific labeling has become possible. In this review, we provide a structured overview of covalent labeling approaches for nucleic acids and highlight notable developments, in particular recent examples. The majority of this review will focus on fluorescent labeling; however, the principles can often be readily applied to other labels. We will start with entirely chemical approaches, followed by chemo-enzymatic strategies and ribozymes, and finish with metabolic labeling of nucleic acids. Each section is subdivided into direct (or one-step) and two-step labeling approaches and will start with DNA before treating RNA.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Mardis ER. Nat Protoc. 2017;12:213–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

