Commercial Serology Assays Predict Neutralization Activity against SARS-CoV-2
- PMID: 33084854
- PMCID: PMC7665417
- DOI: 10.1093/clinchem/hvaa262
Commercial Serology Assays Predict Neutralization Activity against SARS-CoV-2
Abstract
Background: It is unknown whether a positive serology result correlates with protective immunity against SARS-CoV-2. There are also concerns regarding the low positive predictive value of SARS-CoV-2 serology tests, especially when testing populations with low disease prevalence.
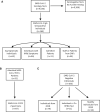
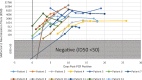
Methods: A neutralization assay was validated in a set of PCR-confirmed positive specimens and in a negative cohort. In addition, 9530 specimens were screened using the Diazyme SARS-CoV-2 IgG serology assay and all positive results (N = 164 individuals) were reanalyzed using the neutralization assay, the Roche total immunoglobin assay, and the Abbott IgG assay. The relationship between the magnitude of a positive SARS-CoV-2 serology result and neutralizing activity was determined. Neutralizing antibody titers (50% inhibitory dilution, ID50) were also longitudinally monitored in patients confirmed to have SARS-CoV-2 by PCR.
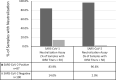
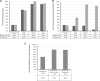
Results: The SARS-CoV-2 neutralization assay had a positive percentage agreement (PPA) of 96.6% with a SARS-CoV-2 PCR test and a negative percentage agreement (NPA) of 98.0% across 100 negative control individuals. ID50 neutralization titers positively correlated with all 3 clinical serology platforms. Longitudinal monitoring of hospitalized PCR-confirmed patients with COVID-19 demonstrated they made high neutralization titers against SARS-CoV-2. PPA between the Diazyme IgG assay alone and the neutralization assay was 50.6%, while combining the Diazyme IgG assay with either the Roche or Abbott platforms increased the PPA to 79.2 and 78.4%, respectively.
Conclusions: These 3 clinical serology assays positively correlate with SARS-CoV-2 neutralization activity observed in patients with COVID-19. All patients confirmed SARS-CoV-2 positive by PCR develop neutralizing antibodies.
Keywords: COVID-19; SARS-CoV-2; immunity; neutralizing antibodies; serology.
© American Association for Clinical Chemistry 2020. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Association between SARS-CoV-2 Neutralizing Antibodies and Commercial Serological Assays.Clin Chem. 2020 Dec 1;66(12):1538-1547. doi: 10.1093/clinchem/hvaa211. Clin Chem. 2020. PMID: 32894750 Free PMC article.
-
Evaluation of a Surrogate Enzyme-Linked Immunosorbent Assay-Based Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) cPass Neutralization Antibody Detection Assay and Correlation With Immunoglobulin G Commercial Serology Assays.Arch Pathol Lab Med. 2021 Oct 1;145(10):1212-1220. doi: 10.5858/arpa.2021-0213-SA. Arch Pathol Lab Med. 2021. PMID: 34181714
-
Multi-Platform Comparison of SARS-CoV-2 Serology Assays for the Detection of COVID-19.J Appl Lab Med. 2020 Nov 1;5(6):1324-1336. doi: 10.1093/jalm/jfaa139. J Appl Lab Med. 2020. PMID: 32766840 Free PMC article.
-
Recent Developments in SARS-CoV-2 Neutralizing Antibody Detection Methods.Curr Med Sci. 2021 Dec;41(6):1052-1064. doi: 10.1007/s11596-021-2470-7. Epub 2021 Dec 21. Curr Med Sci. 2021. PMID: 34935114 Free PMC article. Review.
-
A comparative review of immunoassays for COVID-19 detection.Expert Rev Clin Immunol. 2021 Jun;17(6):573-599. doi: 10.1080/1744666X.2021.1908886. Expert Rev Clin Immunol. 2021. PMID: 33787412 Review.
Cited by
-
An Evaluation of Serological Tests to Determine Postvaccinal Immunity to SARS-CoV-2 by mRNA Vaccines.J Clin Med. 2022 Dec 19;11(24):7534. doi: 10.3390/jcm11247534. J Clin Med. 2022. PMID: 36556149 Free PMC article.
-
Evaluation of post-vaccination immunoglobulin G antibodies and T-cell immune response after inoculation with different types and doses of SARS-CoV-2 vaccines: A retrospective cohort study.Front Med (Lausanne). 2023 Jan 10;9:1092646. doi: 10.3389/fmed.2022.1092646. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36703898 Free PMC article.
-
Assessment of serological assays for identifying high titer convalescent plasma.Transfusion. 2021 Sep;61(9):2658-2667. doi: 10.1111/trf.16580. Epub 2021 Jul 6. Transfusion. 2021. PMID: 34216156 Free PMC article.
-
Prevalence of neutralising antibodies against SARS-CoV-2 in acute infection and convalescence: A systematic review and meta-analysis.PLoS Negl Trop Dis. 2021 Jul 8;15(7):e0009551. doi: 10.1371/journal.pntd.0009551. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34237072 Free PMC article.
-
Performance of the Abbott SARS-CoV-2 IgG serological assay in South African 2 patients.PLoS One. 2022 Feb 4;17(2):e0262442. doi: 10.1371/journal.pone.0262442. eCollection 2022. PLoS One. 2022. PMID: 35120133 Free PMC article.

