Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases
- PMID: 33084883
- PMCID: PMC7578566
- DOI: 10.1042/CS20190893
Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases
Abstract
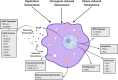
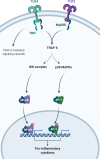
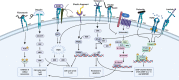
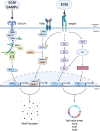
The extracellular matrix (ECM) is a complex network of macromolecules surrounding cells providing structural support and stability to tissues. The understanding of the ECM and the diverse roles it plays in development, homoeostasis and injury have greatly advanced in the last three decades. The ECM is crucial for maintaining tissue homoeostasis but also many pathological conditions arise from aberrant matrix remodelling during ageing. Ageing is characterised as functional decline of tissue over time ultimately leading to tissue dysfunction, and is a risk factor in many diseases including cardiovascular disease, diabetes, cancer, dementia, glaucoma, chronic obstructive pulmonary disease (COPD) and fibrosis. ECM changes are recognised as a major driver of aberrant cell responses. Mesenchymal cells in aged tissue show signs of growth arrest and resistance to apoptosis, which are indicative of cellular senescence. It was recently postulated that cellular senescence contributes to the pathogenesis of chronic fibrotic diseases in the heart, kidney, liver and lung. Senescent cells negatively impact tissue regeneration while creating a pro-inflammatory environment as part of the senescence-associated secretory phenotype (SASP) favouring disease progression. In this review, we explore and summarise the current knowledge around how aberrant ECM potentially influences the senescent phenotype in chronic fibrotic diseases. Lastly, we will explore the possibility for interventions in the ECM-senescence regulatory pathways for therapeutic potential in chronic fibrotic diseases.
Keywords: Antifibrotics; DAMPs; Senescence; Senolytics; extracellular matrix; fibrosis.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

