Mechanisms coordinating ribosomal protein gene transcription in response to stress
- PMID: 33084907
- PMCID: PMC7672434
- DOI: 10.1093/nar/gkaa852
Mechanisms coordinating ribosomal protein gene transcription in response to stress
Abstract
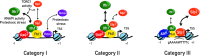
While expression of ribosomal protein genes (RPGs) in the budding yeast has been extensively studied, a longstanding enigma persists regarding their co-regulation under fluctuating growth conditions. Most RPG promoters display one of two distinct arrangements of a core set of transcription factors (TFs) and are further differentiated by the presence or absence of the HMGB protein Hmo1. However, a third group of promoters appears not to be bound by any of these proteins, raising the question of how the whole suite of genes is co-regulated. We demonstrate here that all RPGs are regulated by two distinct, but complementary mechanisms driven by the TFs Ifh1 and Sfp1, both of which are required for maximal expression in optimal conditions and coordinated downregulation upon stress. At the majority of RPG promoters, Ifh1-dependent regulation predominates, whereas Sfp1 plays the major role at all other genes. We also uncovered an unexpected protein homeostasis-dependent binding property of Hmo1 at RPG promoters. Finally, we show that the Ifh1 paralog Crf1, previously described as a transcriptional repressor, can act as a constitutive RPG activator. Our study provides a more complete picture of RPG regulation and may serve as a paradigm for unravelling RPG regulation in multicellular eukaryotes.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

