Expression profiles of metallothionein-I/II and megalin/LRP-2 in uterine cervical squamous lesions
- PMID: 33084977
- PMCID: PMC7990851
- DOI: 10.1007/s00428-020-02947-w
Expression profiles of metallothionein-I/II and megalin/LRP-2 in uterine cervical squamous lesions
Abstract
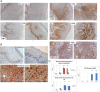
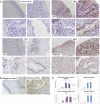
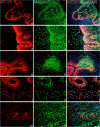
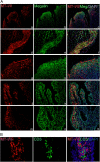
Metallothioneins (MTs) are phylogenetically old cysteine-rich proteins, which are implicated in a variety of physiological and pathological processes. Their growth-regulating, anti-apoptotic and anti-inflammatory functions have been attributed not only to intracellular free radical scavenging and to zinc and copper regulation but also to the ability of secreted MT to bind on surface lipoprotein receptor-megalin/LRP2, which enables the endocytosis of MT-I/II and a wide range of other functionally distinct ligands. In the present study, we analysed the expression pattern of both proteins in 55 cases of premalignant transformation of cervical squamous cells, i.e. in low- and high-grade squamous intraepithelial lesion (LSIL and HSIL). The data showed that in LSIL (cervical intraepithelial neoplasia CIN1; N = 25) MTs were present only in basal and parabasal cells and that megalin was only weakly expressed. In HSIL (CIN2; N = 15 and CIN 3/carcinoma in situ; N = 15), however, overexpression and co-localization of MT with megalin were found in the entire hyperplastic epithelium. Moreover, megalin immunoreactivity appeared on the glandular epithelium and vascular endothelium, as well as on lymphatic cells in stroma. Besides, multiple megalin-positive cells expressed phosphorylated Akt1, implying that MT- and/or megalin-dependent prosurvival signal transduction pathways might contribute to the development of severe cervical dysplasia. The data emphasize the diagnostic power of combined MT/megalin analysis in pre-cancer screening.
Keywords: Akt1/protein kinase B phosphorylation; Biomarkers; CIN lesions, low-density lipoprotein receptor–related protein-2; Metallothionein-I/II; Tumour microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Immunohistochemical detection of metallothionein and MIB1 in uterine cervical squamous lesions.Int J Gynecol Pathol. 1998 Jan;17(1):29-35. doi: 10.1097/00004347-199801000-00006. Int J Gynecol Pathol. 1998. PMID: 9475189
-
Laminin-5 gamma 2 chain expression in cervical intraepithelial neoplasia and invasive cervical carcinoma.Acta Obstet Gynecol Scand. 2005 Nov;84(11):1119-23. doi: 10.1111/j.0001-6349.2005.00879.x. Acta Obstet Gynecol Scand. 2005. PMID: 16232183
-
Stathmin-1 expression as a complement to p16 helps identify high-grade cervical intraepithelial neoplasia with increased specificity.Am J Surg Pathol. 2013 Jan;37(1):89-97. doi: 10.1097/PAS.0b013e3182753f5a. Am J Surg Pathol. 2013. PMID: 23211296
-
Clinical importance of "low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion (LSIL-H)" terminology for cervical smears 5-year analysis of the positive predictive value of LSIL-H compared with ASC-H, LSIL, and HSIL in the detection of high-grade cervical lesions with a review of the literature.Gynecol Oncol. 2011 Apr;121(1):152-6. doi: 10.1016/j.ygyno.2010.12.004. Epub 2011 Jan 5. Gynecol Oncol. 2011. PMID: 21211831 Review.
-
[Remodeling of angiogenesis and lymphangiogenesis in cervical cancer development].Biomed Khim. 2015 Sep-Oct;61(5):579-97. doi: 10.18097/PBMC20156105579. Biomed Khim. 2015. PMID: 26539865 Review. Russian.
Cited by
-
An Enhanced Histopathology Analysis: An AI-Based System for Multiclass Grading of Oral Squamous Cell Carcinoma and Segmenting of Epithelial and Stromal Tissue.Cancers (Basel). 2021 Apr 8;13(8):1784. doi: 10.3390/cancers13081784. Cancers (Basel). 2021. PMID: 33917952 Free PMC article.
-
Metallothioneins and Megalin Expression Profiling in Premalignant and Malignant Oral Squamous Epithelial Lesions.Cancers (Basel). 2021 Sep 9;13(18):4530. doi: 10.3390/cancers13184530. Cancers (Basel). 2021. PMID: 34572758 Free PMC article.
-
Megalin Expression in Primary Oral Squamous Cell Carcinoma Is Associated with the Presence of Lymph Node Metastases, Vascular Invasion, and Lower Overall Survival.Curr Issues Mol Biol. 2023 Mar 24;45(4):2757-2766. doi: 10.3390/cimb45040180. Curr Issues Mol Biol. 2023. PMID: 37185704 Free PMC article.
-
Cleft lip and palate transmembrane protein 1-like is a putative regulator of tumorigenesis and sensitization of cervical cancer cells to cisplatin.Front Oncol. 2024 Sep 13;14:1440906. doi: 10.3389/fonc.2024.1440906. eCollection 2024. Front Oncol. 2024. PMID: 39346724 Free PMC article.
References
-
- Margoshes M, Vallee B. A cadmium protein from equine kidney cortex. J Am Chem Soc. 1957;79:4813–4814. doi: 10.1021/ja01574a064. - DOI

