Aging and chronic high-fat feeding negatively affect kidney size, function, and gene expression in CTRP1-deficient mice
- PMID: 33085906
- PMCID: PMC7847058
- DOI: 10.1152/ajpregu.00139.2020
Aging and chronic high-fat feeding negatively affect kidney size, function, and gene expression in CTRP1-deficient mice
Abstract
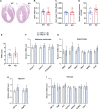
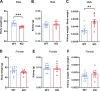
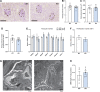
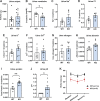
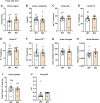
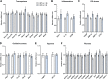
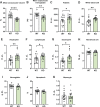
C1q/TNF-related protein 1 (CTRP1) is an endocrine factor with metabolic, cardiovascular, and renal functions. We previously showed that aged Ctrp1-knockout (KO) mice fed a control low-fat diet develop renal hypertrophy and dysfunction. Since aging and obesity adversely affect various organ systems, we hypothesized that aging, in combination with obesity induced by chronic high-fat feeding, would further exacerbate renal dysfunction in CTRP1-deficient animals. To test this, we fed wild-type and Ctrp1-KO mice a high-fat diet for 8 mo or longer. Contrary to our expectation, no differences were observed in blood pressure, heart function, or vascular stiffness between genotypes. Loss of CTRP1, however, resulted in an approximately twofold renal enlargement (relative to body weight), ∼60% increase in urinary total protein content, and elevated pH, and changes in renal gene expression affecting metabolism, signaling, transcription, cell adhesion, solute and metabolite transport, and inflammation. Assessment of glomerular integrity, the extent of podocyte foot process effacement, as well as renal response to water restriction and salt loading did not reveal significant differences between genotypes. Interestingly, blood platelet, white blood cell, neutrophil, lymphocyte, and eosinophil counts were significantly elevated, whereas mean corpuscular volume and hemoglobin were reduced in Ctrp1-KO mice. Cytokine profiling revealed increased circulating levels of CCL17 and TIMP-1 in KO mice. Compared with our previous study, current data suggest that chronic high-fat feeding affects renal phenotypes differently than similarly aged mice fed a control low-fat diet, highlighting a diet-dependent contribution of CTRP1 deficiency to age-related changes in renal structure and function.
Keywords: aging; heart; kidney; metabolism; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Late-onset renal hypertrophy and dysfunction in mice lacking CTRP1.FASEB J. 2020 Feb;34(2):2657-2676. doi: 10.1096/fj.201900558RR. Epub 2019 Dec 26. FASEB J. 2020. PMID: 31908037 Free PMC article.
-
Loss of CTRP1 disrupts glucose and lipid homeostasis.Am J Physiol Endocrinol Metab. 2016 Oct 1;311(4):E678-E697. doi: 10.1152/ajpendo.00087.2016. Epub 2016 Aug 23. Am J Physiol Endocrinol Metab. 2016. PMID: 27555298 Free PMC article.
-
CTRP1 Attenuates UUO-induced Renal Fibrosis via AMPK/NOX4 Pathway in Mice.Curr Med Sci. 2020 Feb;40(1):48-54. doi: 10.1007/s11596-020-2145-9. Epub 2020 Mar 13. Curr Med Sci. 2020. PMID: 32166664
-
CTRP1 prevents high fat diet-induced obesity and improves glucose homeostasis in obese and STZ-induced diabetic mice.J Transl Med. 2022 Oct 4;20(1):449. doi: 10.1186/s12967-022-03672-5. J Transl Med. 2022. PMID: 36195912 Free PMC article.
-
C1q/TNF-related Protein 1, a Multifunctional Adipokine: An Overview of Current Data.Am J Med Sci. 2020 Sep;360(3):222-228. doi: 10.1016/j.amjms.2020.05.036. Epub 2020 May 28. Am J Med Sci. 2020. PMID: 32591091 Review.
Cited by
-
CTRP11 contributes modestly to systemic metabolism and energy balance.FASEB J. 2022 Jun;36(6):e22347. doi: 10.1096/fj.202200189RR. FASEB J. 2022. PMID: 35579659 Free PMC article.
-
Loss of CTRP10 results in female obesity with preserved metabolic health.bioRxiv [Preprint]. 2025 Jan 31:2023.11.01.565163. doi: 10.1101/2023.11.01.565163. bioRxiv. 2025. Update in: Elife. 2025 Mar 24;13:RP93373. doi: 10.7554/eLife.93373. PMID: 37961647 Free PMC article. Updated. Preprint.
-
Role of CTRP14/C1QL1 in motor coordination and learning across the lifespan.Physiol Behav. 2025 Mar 15;291:114799. doi: 10.1016/j.physbeh.2025.114799. Epub 2025 Jan 4. Physiol Behav. 2025. PMID: 39761721
-
Loss of CTRP10 results in female obesity with preserved metabolic health.Elife. 2025 Mar 24;13:RP93373. doi: 10.7554/eLife.93373. Elife. 2025. PMID: 40126547 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

