Guidance for Restarting Inflammatory Bowel Disease Therapy in Patients Who Withheld Immunosuppressant Medications During COVID-19
- PMID: 33085972
- PMCID: PMC7665410
- DOI: 10.1093/ecco-jcc/jjaa135
Guidance for Restarting Inflammatory Bowel Disease Therapy in Patients Who Withheld Immunosuppressant Medications During COVID-19
Abstract
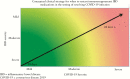
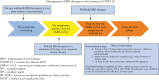
Patients with inflammatory bowel diseases [IBD] are frequently treated with immunosuppressant medications. During the coronavirus disease 2019 [COVID-19] pandemic, recommendations for IBD management have included that patients should stay on their immunosuppressant medications if they are not infected with the severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2], but to temporarily hold these medications if symptomatic with COVID-19 or asymptomatic but have tested positive for SARS-CoV-2. As more IBD patients are infected globally, it is important to also understand how to manage IBD medications during convalescence while an individual with IBD is recovering from COVID-19. In this review, we address the differences between a test-based versus a symptoms-based strategy as related to COVID-19, and offer recommendations on when it is appropriate to consider restarting IBD therapy in patients testing positive for SARS-CoV-2 or with clinical symptoms consistent with COVID-19. In general, we recommend a symptoms-based approach, due to the current lack of confidence in the accuracy of available testing and the clinical significance of prolonged detection of virus via molecular testing.
Keywords: Crohn’s; IBD; biologic; de-escalation; immunomodulator.
© The Author(s) 2020. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous