Continuous-Flow Production of Perfluorocarbon-Loaded Polymeric Nanoparticles: From the Bench to Clinic
- PMID: 33086007
- PMCID: PMC7645868
- DOI: 10.1021/acsami.0c12020
Continuous-Flow Production of Perfluorocarbon-Loaded Polymeric Nanoparticles: From the Bench to Clinic
Abstract
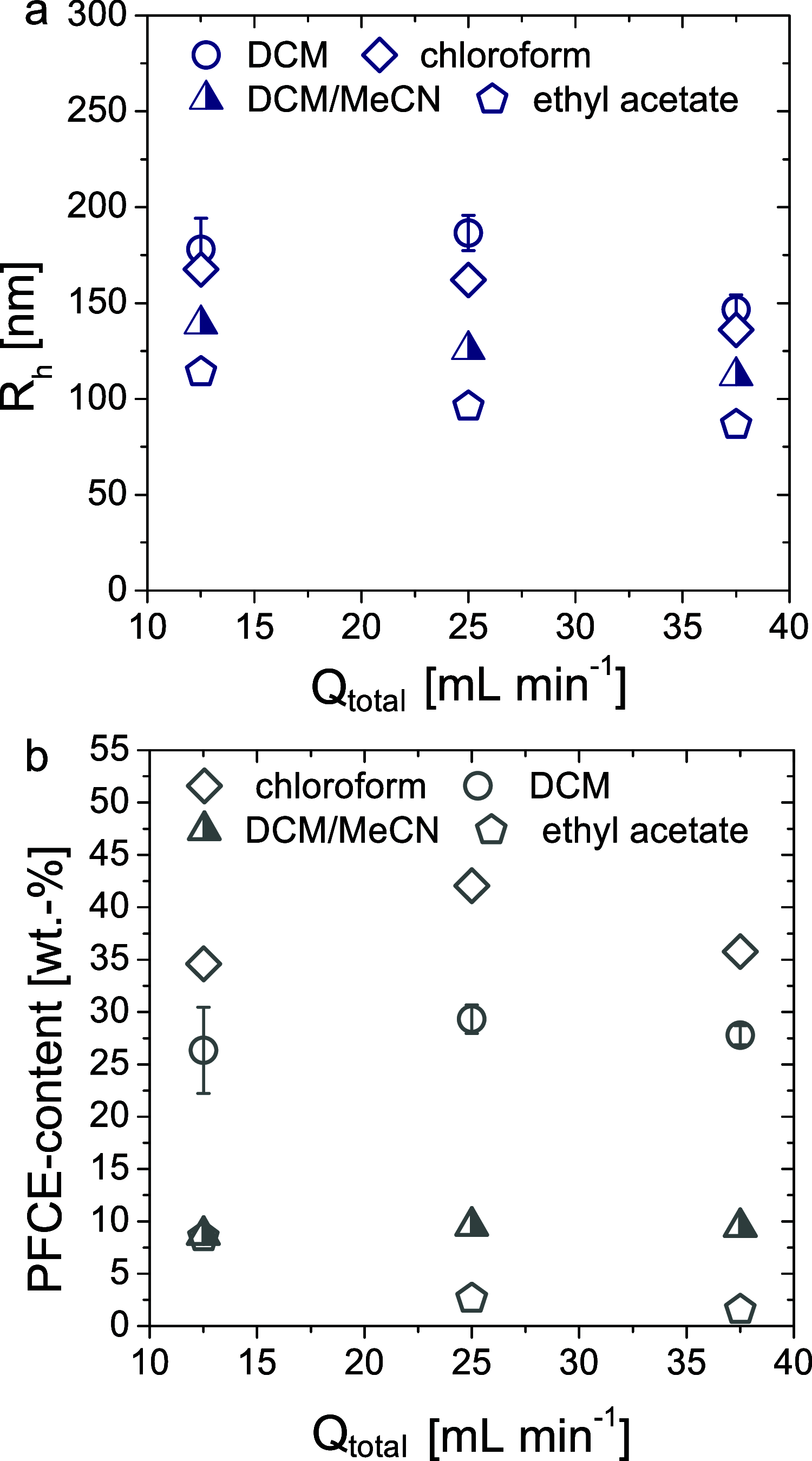
Perfluorocarbon-loaded nanoparticles are powerful theranostic agents, which are used in the therapy of cancer and stroke and as imaging agents for ultrasound and 19F magnetic resonance imaging (MRI). Scaling up the production of perfluorocarbon-loaded nanoparticles is essential for clinical translation. However, it represents a major challenge as perfluorocarbons are hydrophobic and lipophobic. We developed a method for continuous-flow production of perfluorocarbon-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles using a modular microfluidic system, with sufficient yields for clinical use. We combined two slit interdigital micromixers with a sonication flow cell to achieve efficient mixing of three phases: liquid perfluorocarbon, PLGA in organic solvent, and aqueous surfactant solution. The production rate was at least 30 times higher than with the conventional formulation. The characteristics of nanoparticles can be adjusted by changing the flow rates and type of solvent, resulting in a high PFC loading of 20-60 wt % and radii below 200 nm. The nanoparticles are nontoxic, suitable for 19F MRI and ultrasound imaging, and can dissolve oxygen. In vivo 19F MRI with perfluoro-15-crown-5 ether-loaded nanoparticles showed similar biodistribution as nanoparticles made with the conventional method and a fast clearance from the organs. Overall, we developed a continuous, modular method for scaled-up production of perfluorocarbon-loaded nanoparticles that can be potentially adapted for the production of other multiphase systems. Thus, it will facilitate the clinical translation of theranostic agents in the future.
Keywords: 19F MRI; cell tracking; microfluidics; multimodal imaging; nanoparticles; oxygen carriers; perfluorocarbon; ultrasound.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Liu J.; Chen Q.; Feng L.; Liu Z. Nanomedicine for Tumor Microenvironment Modulation and Cancer Treatment Enhancement. Nano Today 2018, 21, 55–73. 10.1016/j.nantod.2018.06.008. - DOI
-
- Hu D.; Zhong L.; Wang M.; Li H.; Qu Y.; Liu Q.; Han R.; Yuan L.; Shi K.; Peng J.; Qian Z. Perfluorocarbon-Loaded and Redox-Activatable Photosensitizing Agent with Oxygen Supply for Enhancement of Fluorescence/Photoacoustic Imaging Guided Tumor Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1806199. 10.1002/adfm.201806199. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

