Molecular Aspects of Thyroid Calcification
- PMID: 33086487
- PMCID: PMC7589718
- DOI: 10.3390/ijms21207718
Molecular Aspects of Thyroid Calcification
Abstract
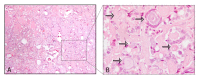
In thyroid cancer, calcification is mainly present in classical papillary thyroid carcinoma (PTC) and in medullary thyroid carcinoma (MTC), despite being described in benign lesions and in other subtypes of thyroid carcinomas. Thyroid calcifications are classified according to their diameter and location. At ultrasonography, microcalcifications appear as hyperechoic spots ≤ 1 mm in diameter and can be named as stromal calcification, bone formation, or psammoma bodies (PBs), whereas calcifications > 1 mm are macrocalcifications. The mechanism of their formation is still poorly understood. Microcalcifications are generally accepted as a reliable indicator of malignancy as they mostly represent PBs. In order to progress in terms of the understanding of the mechanisms behind calcification occurring in thyroid tumors in general, and in PTC in particular, we decided to use histopathology as the basis of the possible cellular and molecular mechanisms of calcification formation in thyroid cancer. We explored the involvement of molecules such as runt-related transcription factor-2 (Runx-2), osteonectin/secreted protein acidic and rich in cysteine (SPARC), alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteopontin (OPN) in the formation of calcification. The present review offers a novel insight into the mechanisms underlying the development of calcification in thyroid cancer.
Keywords: calcifications; osteopontin; psammoma bodies; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Cooper D.S., Doherty G.M., Haugen B.R., Kloos R.T., Lee S.L., Mandel S.J., Mazzaferri E.L., McIver B., Pacini F., Schlumberger M., et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. - DOI - PubMed
-
- Singer P.A., Cooper D.S., Daniels G.H., Ladenson P.W., Greenspan F.S., Levy E.G., Braverman L.E., Clark O.H., McDougall I.R., Ain K.V., et al. Treatment Guidelines for Patients With Thyroid Nodules and Well-Differentiated Thyroid Cancer. Arch. Intern. Med. 1996;156:2165–2172. doi: 10.1001/archinte.1996.00440180017002. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- POCI-01-0145-FEDER-016390: CANCEL STEM/European Regional Development Fund
- POCI-01-0145-FEDER-007274/Fundação para a Ciência e a Tecnologia
- POCI-01-0145-FEDER-031438/Fundação para a Ciência e a Tecnologia
- PDTC/MED_ONC/31438/2017/Fundação para a Ciência e a Tecnologia
- CEECIND/00201/2017/Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

