Phosphatase and Tensin Homolog (PTEN) of Japanese Flounder-Its Regulation by miRNA and Role in Autophagy, Apoptosis and Pathogen Infection
- PMID: 33086544
- PMCID: PMC7589652
- DOI: 10.3390/ijms21207725
Phosphatase and Tensin Homolog (PTEN) of Japanese Flounder-Its Regulation by miRNA and Role in Autophagy, Apoptosis and Pathogen Infection
Abstract
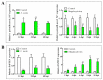
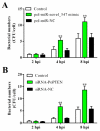
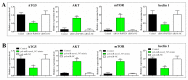
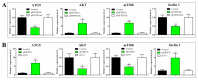
MicroRNAs (miRNAs) are small non-coding RNAs with important roles in diverse biological processes including immunity. Japanese flounder (Paralichthys olivaceus) is an aquaculture fish species susceptible to the infection of bacterial and viral pathogens including Edwardsiella tarda. In a previous study, pol-miR-novel_547, a novel miRNA of flounder with unknown function, was found to be induced by E. tarda. In the present study, we investigated the regulation and function of pol-miR-novel_547 and its target gene. We found that pol-miR-novel_547 was regulated differently by E. tarda and the viral pathogen megalocytivirus, and pol-miR-novel_547 repressed the expression of PTEN (phosphatase and tensin homolog) of flounder (PoPTEN). PoPTEN is ubiquitously expressed in multiple tissues of flounder and responded to bacterial and viral infections. Interference with PoPTEN expression in flounder cells directly or via pol-miR-novel_547 promoted E. tarda invasion. Consistently, in vivo knockdown of PoPTEN enhanced E. tarda dissemination in flounder tissues, whereas in vivo overexpression of PoPTEN attenuated E. tarda dissemination but facilitated megalocytivirus replication. Further in vitro and in vivo studies showed that PoPTEN affected autophagy activation via the AKT/mTOR pathway and also modulated the process of apoptosis. Together these results reveal for the first time a critical role of fish PTEN and its regulatory miRNA in pathogen infection, autophagy, and apoptosis.
Keywords: Paralichthys olivaceus; apoptosis; autophagy; immune defense; microRNA; pathogen infection; phosphatase and tensin homolog.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Wynendaele J., Böhnke A., Leucci E., Nielsen S.J., Lambertz I., Hammer S., Sbrzesny N., Kubitza D., Wolf A., Gradhand E., et al. An illegitimate microRNA target site within the 3′ UTR of MDM4 affects ovarian cancer progression and chemosensitivity. Cancer Res. 2010;70:9641–9649. doi: 10.1158/0008-5472.CAN-10-0527. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

