Label-Free Optical Resonator-Based Biosensors
- PMID: 33086566
- PMCID: PMC7589515
- DOI: 10.3390/s20205901
Label-Free Optical Resonator-Based Biosensors
Abstract
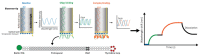
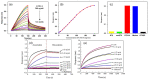
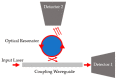
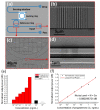
The demand for biosensor technology has grown drastically over the last few decades, mainly in disease diagnosis, drug development, and environmental health and safety. Optical resonator-based biosensors have been widely exploited to achieve highly sensitive, rapid, and label-free detection of biological analytes. The advancements in microfluidic and micro/nanofabrication technologies allow them to be miniaturized and simultaneously detect various analytes in a small sample volume. By virtue of these advantages and advancements, the optical resonator-based biosensor is considered a promising platform not only for general medical diagnostics but also for point-of-care applications. This review aims to provide an overview of recent progresses in label-free optical resonator-based biosensors published mostly over the last 5 years. We categorized them into Fabry-Perot interferometer-based and whispering gallery mode-based biosensors. The principles behind each biosensor are concisely introduced, and recent progresses in configurations, materials, test setup, and light confinement methods are described. Finally, the current challenges and future research topics of the optical resonator-based biosensor are discussed.
Keywords: Fabry-Perot interferometer; label-free biosensors; optical resonators; whispering gallery mode resonators.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


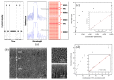
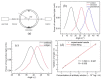

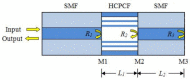

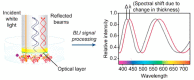







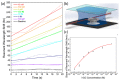
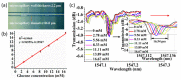
Similar articles
-
Label-Free Biological and Chemical Sensing Using Whispering Gallery Mode Optical Resonators: Past, Present, and Future.Sensors (Basel). 2017 Mar 8;17(3):540. doi: 10.3390/s17030540. Sensors (Basel). 2017. PMID: 28282881 Free PMC article. Review.
-
Whispering-Gallery Mode Resonators for Detecting Cancer.Sensors (Basel). 2017 Sep 13;17(9):2095. doi: 10.3390/s17092095. Sensors (Basel). 2017. PMID: 28902169 Free PMC article. Review.
-
A review of specialty fiber biosensors based on interferometer configuration.J Biophotonics. 2021 Jun;14(6):e202100068. doi: 10.1002/jbio.202100068. Epub 2021 Apr 30. J Biophotonics. 2021. PMID: 33797865 Review.
-
Applications of Optical Fiber in Label-Free Biosensors and Bioimaging: A Review.Biosensors (Basel). 2022 Dec 30;13(1):64. doi: 10.3390/bios13010064. Biosensors (Basel). 2022. PMID: 36671899 Free PMC article. Review.
-
Towards next-generation label-free biosensors: recent advances in whispering gallery mode sensors.Lab Chip. 2017 Mar 29;17(7):1190-1205. doi: 10.1039/c6lc01595f. Lab Chip. 2017. PMID: 28265608 Review.
Cited by
-
Development of Methods for Specific Capture of Biological Targets on Aluminum Substrates: Application to Bacillus subtilis Spore Detection as a Model for Anthrax.Sensors (Basel). 2022 Apr 30;22(9):3441. doi: 10.3390/s22093441. Sensors (Basel). 2022. PMID: 35591130 Free PMC article.
-
Cancer treatment approaches within the frame of hyperthermia, drug delivery systems, and biosensors: concepts and future potentials.RSC Adv. 2024 Dec 12;14(53):39297-39324. doi: 10.1039/d4ra06992g. eCollection 2024 Dec 10. RSC Adv. 2024. PMID: 39670162 Free PMC article. Review.
-
Multimode sensing based on optical microcavities.Front Optoelectron. 2023 Oct 27;16(1):29. doi: 10.1007/s12200-023-00084-1. Front Optoelectron. 2023. PMID: 37889446 Free PMC article. Review.
-
Label-Free Physical Techniques and Methodologies for Proteins Detection in Microfluidic Biosensor Structures.Biomedicines. 2022 Jan 18;10(2):207. doi: 10.3390/biomedicines10020207. Biomedicines. 2022. PMID: 35203416 Free PMC article. Review.
-
Critically coupled Fabry-Perot cavity with high signal contrast for refractive index sensing.Sci Rep. 2021 Oct 1;11(1):19575. doi: 10.1038/s41598-021-98654-w. Sci Rep. 2021. PMID: 34599221 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

