Updating Phospholipase A2 Biology
- PMID: 33086624
- PMCID: PMC7603386
- DOI: 10.3390/biom10101457
Updating Phospholipase A2 Biology
Abstract
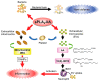
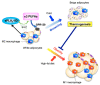
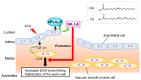
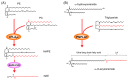
The phospholipase A2 (PLA2) superfamily contains more than 50 enzymes in mammals that are subdivided into several distinct families on a structural and biochemical basis. In principle, PLA2 has the capacity to hydrolyze the sn-2 position of glycerophospholipids to release fatty acids and lysophospholipids, yet several enzymes in this superfamily catalyze other reactions rather than or in addition to the PLA2 reaction. PLA2 enzymes play crucial roles in not only the production of lipid mediators, but also membrane remodeling, bioenergetics, and body surface barrier, thereby participating in a number of biological events. Accordingly, disturbance of PLA2-regulated lipid metabolism is often associated with various diseases. This review updates the current state of understanding of the classification, enzymatic properties, and biological functions of various enzymes belonging to the PLA2 superfamily, focusing particularly on the novel roles of PLA2s in vivo.
Keywords: fatty acid; knockout mouse; lipid mediator; lipidomics; lysophospholipid; membrane; phospholipase A2; phospholipid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

