Analysis of Amygdalin in Various Matrices Using Electrospray Ionization and Flowing Atmospheric-Pressure Afterglow Mass Spectrometry
- PMID: 33086630
- PMCID: PMC7603377
- DOI: 10.3390/biom10101459
Analysis of Amygdalin in Various Matrices Using Electrospray Ionization and Flowing Atmospheric-Pressure Afterglow Mass Spectrometry
Abstract
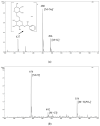
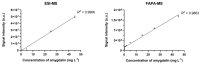
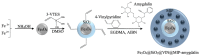
Amygdalin is a natural cyanogenic compound that plants produce in the fight against insects and herbivores. Excessive amounts of amygdalin by animals and humans can potentially lead to fatal intoxication. However, studies confirm that amygdalin has antitumor properties, including the ability to inhibit the proliferation of cancer cells and to induce their apoptosis. The analysis of amygdalin in various matrices is an important analytical problem today. The publication presents the methodology of direct determination of amygdalin in water, sewage, and biological materials using electrospray ionization mass spectrometry (ESI-MS) and a new analytical method using flowing atmospheric-pressure afterglow mass spectrometry (FAPA-MS). The methods of analyte pre-concentration using a magnetic, molecularly imprinted polymer (mag-MIP) and the influence of interferents on the recorded spectra were discussed. Analytical parameters in ESI-MS and FAPA-MS methods were established. The linearity range was 4.5 µg L-1-45 mg L-1 in positive mode ESI-MS and FAPA-MS. The limit of detection (LOD) for ESI-MS was 0.101 ± 0.003 µg L-1 and the limit of quantification (LOQ) was 0.303 ± 0.009 µg L-1. In FAPA-MS, the LOD was 0.050 ± 0.002 µg L-1 and the LOQ was 0.150 ± 0.006 µg L-1. The content of amygdalin in various matrices was determined.
Keywords: ESI-MS; FAPA-MS; amygdalin; mag-MIP.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



















References
-
- Cairns T., Froberg J.E., Gonzales S., Langham W.S., Stamp J.J., Howie J.K., Sawyer D.T. Analytical Chemistry of Amygdalin. Anal. Chem. 1978;50:317–322. doi: 10.1021/ac50024a037. - DOI
-
- Akah P., Alemji J. Studies on the effects of Vernonia amygdalina leaf extract and fractions on biochemical and hematological parameters in diabetic rats. Planta Med. 2009:75–82. doi: 10.1055/s-0029-1234707. - DOI
-
- Alara O.R., Kholijah S., Mudalip A., Olalere O.A. Phytochemical And Pharmacological Properties Of Vernonia amygdalina: A Review Ultrasonic assisted crystallization process View project Biological activity of mushrooms extracts adsorbed on SZN View project. J. Chem. Eng. Ind. Biotechnol. Open Access. 2017;2:80–96.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

