Immunological Aspects of Chytridiomycosis
- PMID: 33086692
- PMCID: PMC7712659
- DOI: 10.3390/jof6040234
Immunological Aspects of Chytridiomycosis
Abstract
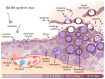
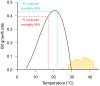
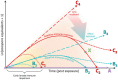
Amphibians are currently the most threatened vertebrate class, with the disease chytridiomycosis being a major contributor to their global declines. Chytridiomycosis is a frequently fatal skin disease caused by the fungal pathogens Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal). The severity and extent of the impact of the infection caused by these pathogens across modern Amphibia are unprecedented in the history of vertebrate infectious diseases. The immune system of amphibians is thought to be largely similar to that of other jawed vertebrates, such as mammals. However, amphibian hosts are both ectothermic and water-dependent, which are characteristics favouring fungal proliferation. Although amphibians possess robust constitutive host defences, Bd/Bsal replicate within host cells once these defences have been breached. Intracellular fungal localisation may contribute to evasion of the induced innate immune response. Increasing evidence suggests that once the innate defences are surpassed, fungal virulence factors suppress the targeted adaptive immune responses whilst promoting an ineffectual inflammatory cascade, resulting in immunopathology and systemic metabolic disruption. Thus, although infections are contained within the integument, crucial homeostatic processes become compromised, leading to mortality. In this paper, we present an integrated synthesis of amphibian post-metamorphic immunological responses and the corresponding outcomes of infection with Bd, focusing on recent developments within the field and highlighting future directions.
Keywords: Batrachochytrium dendrobatidis; Batrachochytrium salamandrivorans; adaptive; amphibian; chytridiomycosis; constitutive; disease; immunopathology; immunosuppression; innate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Berger L., Speare R., Daszak P., Green D.E., Cunningham A.A., Goggin C.L., Slocombe R., Ragan M.A., Hyatt A.D., McDonald K.R., et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. - DOI - PMC - PubMed
-
- Longcore J.E., Pessier A.P., Nichols D.K. Batrochochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. doi: 10.1080/00275514.1999.12061011. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

