Expression Profile of Porcine TRIM26 and Its Inhibitory Effect on Interferon-β Production and Antiviral Response
- PMID: 33086712
- PMCID: PMC7589756
- DOI: 10.3390/genes11101226
Expression Profile of Porcine TRIM26 and Its Inhibitory Effect on Interferon-β Production and Antiviral Response
Abstract
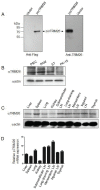
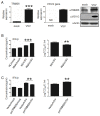
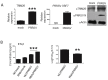
TRIM26, a member of the tripartite motif (TRIM) family has been shown to be involved in modulation of innate antiviral response. However, the functional characteristics of porcine TRIM26 (porTRIM26) are unclear. In this study, we used a synthesized antigen peptide to generate a polyclonal antibody against porTRIM26 with which to study the expression and function of porTRIM26. We demonstrated that polyinosinic:polycytidylic acid (poly (I:C)) stimulation and viral infection (vesicular stomatitis (VSV) or porcine reproductive and respiratory syndrome virus (PRRSV)) induce expression of porTRIM26, whereas knock-down expression of porTRIM26 promotes interferon (IFN)- production after poly (I:C) stimulation and virus infection (VSV or PRRSV). The importance of the porTRIM26-mediated modulation of the antiviral response was also shown in VSV- or PRRSV-infected cells. In summary, these findings show that porTRIM26 has an inhibitory role in IFN- expression and the antiviral response.
Keywords: IFN-; PRRSV; TRIM26; VSV; antiviral response; poly (I:C).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Kimsa M.W., Strzalka-Mrozik B., Kimsa M.C., Mazurek U., Kruszniewska-Rajs C., Gola J., Adamska J., Twardoch M. Differential expression of tripartite motif-containing family in normal human dermal fibroblasts in response to porcine endogenous retrovirus infection. Folia Biol. 2014;60:144–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

