Clock-Controlled Mitochondrial Dynamics Correlates with Cyclic Pregnenolone Synthesis
- PMID: 33086741
- PMCID: PMC7589815
- DOI: 10.3390/cells9102323
Clock-Controlled Mitochondrial Dynamics Correlates with Cyclic Pregnenolone Synthesis
Abstract
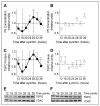
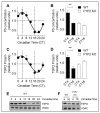
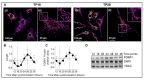
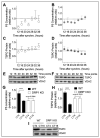
Neurosteroids are steroids synthetized in the nervous system, with the first step of steroidogenesis taking place within mitochondria with the synthesis of pregnenolone. They exert important brain-specific functions by playing a role in neurotransmission, learning and memory processes, and neuroprotection. Here, we show for the first time that mitochondrial neurosteroidogenesis follows a circadian rhythm and correlates with the rhythmic changes in mitochondrial morphology. We used synchronized human A172 glioma cells, which are steroidogenic cells with a functional core molecular clock, to show that pregnenolone levels and translocator protein (TSPO) are controlled by the clock, probably via circadian regulation of mitochondrial fusion/fission. Key findings were recapitulated in mouse brains. We also showed that genetic or pharmacological abrogation of fusion/fission activity, as well as disturbing the core molecular clock, abolished circadian rhythms of pregnenolone and TSPO. Our findings provide new insights into the crosstalk between mitochondrial function (here, neurosteroidogenesis) and circadian cycles.
Keywords: circadian clock; mitochondrial dynamics; neurosteroid; pregnenolone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TSPO Ligands Boost Mitochondrial Function and Pregnenolone Synthesis.J Alzheimers Dis. 2019;72(4):1045-1058. doi: 10.3233/JAD-190127. J Alzheimers Dis. 2019. PMID: 31256132 Free PMC article.
-
Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics.Cell Metab. 2018 Mar 6;27(3):657-666.e5. doi: 10.1016/j.cmet.2018.01.011. Epub 2018 Feb 22. Cell Metab. 2018. PMID: 29478834
-
Steroid hormone synthesis in mitochondria.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):62-73. doi: 10.1016/j.mce.2013.04.014. Epub 2013 Apr 28. Mol Cell Endocrinol. 2013. PMID: 23628605
-
Translocator protein as a promising target for novel anxiolytics.Curr Top Med Chem. 2012;12(4):270-85. doi: 10.2174/156802612799078720. Curr Top Med Chem. 2012. PMID: 22204481 Review.
-
Channel-like functions of the 18-kDa translocator protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response.Curr Pharm Des. 2007;13(23):2385-405. doi: 10.2174/138161207781368710. Curr Pharm Des. 2007. PMID: 17692008 Review.
Cited by
-
Alterations in Pregnenolone and Testosterone Levels in Male Shift Workers.Int J Environ Res Public Health. 2023 Feb 11;20(4):3195. doi: 10.3390/ijerph20043195. Int J Environ Res Public Health. 2023. PMID: 36833889 Free PMC article.
-
Hormonal Regulation of Oxidative Phosphorylation in the Brain in Health and Disease.Cells. 2021 Oct 28;10(11):2937. doi: 10.3390/cells10112937. Cells. 2021. PMID: 34831160 Free PMC article. Review.
-
Unveiling therapeutic targets and preventive components for kidney insufficiency and blood stasis-type BPH: bridging metabolomics, network pharmacology and reverse screening.Front Pharmacol. 2025 Jun 19;16:1584766. doi: 10.3389/fphar.2025.1584766. eCollection 2025. Front Pharmacol. 2025. PMID: 40626307 Free PMC article.
-
Biomaterials-based anti-inflammatory treatment strategies for Alzheimer's disease.Neural Regen Res. 2024 Jan;19(1):100-115. doi: 10.4103/1673-5374.374137. Neural Regen Res. 2024. PMID: 37488851 Free PMC article. Review.
-
Molecular mechanisms of tumour development in glioblastoma: an emerging role for the circadian clock.NPJ Precis Oncol. 2024 Feb 20;8(1):40. doi: 10.1038/s41698-024-00530-z. NPJ Precis Oncol. 2024. PMID: 38378853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

