In Vivo, In Vitro and In Silico Studies of the Hybrid Compound AA3266, an Opioid Agonist/NK1R Antagonist with Selective Cytotoxicity
- PMID: 33086743
- PMCID: PMC7588979
- DOI: 10.3390/ijms21207738
In Vivo, In Vitro and In Silico Studies of the Hybrid Compound AA3266, an Opioid Agonist/NK1R Antagonist with Selective Cytotoxicity
Abstract
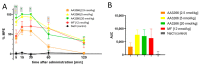
AA3266 is a hybrid compound consisting of opioid receptor agonist and neurokinin-1 receptor (NK1R) antagonist pharmacophores. It was designed with the desire to have an analgesic molecule with improved properties and auxiliary anticancer activity. Previously, the compound was found to exhibit high affinity for μ- and δ-opioid receptors, while moderate binding to NK1R. In the presented contribution, we report on a deeper investigation of this hybrid. In vivo, we have established that AA3266 has potent antinociceptive activity in acute pain model, comparable to that of morphine. Desirably, with prolonged administration, our hybrid induces less tolerance than morphine does. AA3266, contrary to morphine, does not cause development of constipation, which is one of the main undesirable effects of opioid use. In vitro, we have confirmed relatively strong cytotoxic activity on a few selected cancer cell lines, similar to or greater than that of a reference NK1R antagonist, aprepitant. Importantly, our compound affects normal cells to smaller extent what makes our compound more selective against cancer cells. In silico methods, including molecular docking, molecular dynamics simulations and fragment molecular orbital calculations, have been used to investigate the interactions of AA3266 with MOR and NK1R. Insights from these will guide structural optimization of opioid/antitachykinin hybrid compounds.
Keywords: NK1 receptor antagonists; cytotoxicity; fragment molecular orbitals; melanoma; molecular dynamics; morphine; multitarget ligands; opioid; pain; tolerance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

