Endostar continuous versus intermittent intravenous infusion combined with chemotherapy for advanced NSCLC: a systematic review and meta-analysis including non-randomized studies
- PMID: 33087103
- PMCID: PMC7579986
- DOI: 10.1186/s12885-020-07527-4
Endostar continuous versus intermittent intravenous infusion combined with chemotherapy for advanced NSCLC: a systematic review and meta-analysis including non-randomized studies
Abstract
Background: Both intermittent intravenous (IIV) infusion and continuous intravenous (CIV) infusion of Endostar are widely used for NSCLC in China. We aimed to compare the efficacy and safety of CIV of Endostar versus IIV in combination with first-line chemotherapy for patients with advanced NSCLC.
Methods: RCTs, NRCTs and cohort studies which compared CIV of Endostar with IIV in advanced NSCLC patients and reported efficacy or safety outcomes were eligible. Two reviewers independently screened records, extracted data and assessed risk of bias. Pooled risk ratios (RRs) with 95% confidence intervals were calculated using random effects meta-analysis for short-term efficacy and safety outcomes, and hazard ratios (HRs) for survival outcomes.
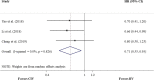
Results: Finally nine studies involving 597 patients were included, containing two RCTs, three NRCTs and four cohort studies. For short-term efficacy, moderate quality of evidence showed that there were no significant differences between CIV of Endostar and IIV in objective response rate (ORR; RR 1.34, 95% CI 0.91-1.98, P = 0.14) and disease control rate (DCR; RR 1.11, 95% CI 0.94-1.30, P = 0.21). Very low quality of evidence indicated that CIV of Endostar significantly improved both overall survival (OS; HR 0.69, 95% CI 0.48-0.99, P = 0.046) and progression-free survival (PFS; HR 0.71, 95% CI 0.55-0.93, P = 0.01) compared with IIV. As for safety outcomes, moderate quality of evidence found that CIV of Endostar significantly reduced the risk of myelosuppression (RR 0.55, 95% CI 0.32-0.96, P = 0.03) and cardiovascular toxicity (RR 0.21, 95% CI 0.06-0.78, P = 0.02) compared with IIV.
Conclusions: In advanced NSCLC, compared with IIV, CIV of Endostar had similar short-term efficacy, and substantially lower risk of myelosuppression and cardiovascular toxicity. Although very low quality of evidence supported the survival benefit of CIV compared with IIV, large RCTs with long-term follow-up are needed to demonstrate survival benefits. Caution should be given for off-label use of CIV of Endostar.
Keywords: Endostar; Meta-analysis; Non-randomized studies; Non-small cell lung cancer; Recombinant human endostatin; Systematic review.
Conflict of interest statement
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. The authors declare that they have no conflicts of interest.
Figures




Similar articles
-
The Evaluation of Durative Transfusion of Endostar Combined with Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer.Chemotherapy. 2018;63(4):214-219. doi: 10.1159/000493098. Epub 2018 Oct 22. Chemotherapy. 2018. PMID: 30347389
-
Different administration routes of recombinant human endostatin combined with concurrent chemoradiotherapy might lead to different efficacy and safety profile in unresectable stage III non-small cell lung cancer: Updated follow-up results from two phase II trials.Thorac Cancer. 2020 Apr;11(4):898-906. doi: 10.1111/1759-7714.13333. Epub 2020 Feb 18. Thorac Cancer. 2020. PMID: 32068962 Free PMC article. Clinical Trial.
-
Comparison of Endostar continuous versus intermittent intravenous infusion in combination with first-line chemotherapy in patients with advanced non-small cell lung cancer.Thorac Cancer. 2019 Jul;10(7):1576-1580. doi: 10.1111/1759-7714.13106. Epub 2019 Jun 3. Thorac Cancer. 2019. PMID: 31161695 Free PMC article.
-
Systematic review and meta-analysis of Endostar (rh-endostatin) combined with chemotherapy versus chemotherapy alone for treating advanced non-small cell lung cancer.World J Surg Oncol. 2012 Aug 24;10:170. doi: 10.1186/1477-7819-10-170. World J Surg Oncol. 2012. PMID: 22917490 Free PMC article.
-
Endostar (rh-endostatin) improves efficacy of concurrent chemoradiotherapy for locally advanced non-small cell lung cancer: A systematic review and meta-analysis.Thorac Cancer. 2021 Dec;12(23):3208-3215. doi: 10.1111/1759-7714.14188. Epub 2021 Oct 21. Thorac Cancer. 2021. PMID: 34676669 Free PMC article.
Cited by
-
Systematic Evaluation Meta-Analysis of the Efficacy of Recombinant Human Endostatin Combined with Gemcitabine and Cisplatin in Non-Small-Cell Lung Cancer.J Healthc Eng. 2022 Mar 15;2022:3208780. doi: 10.1155/2022/3208780. eCollection 2022. J Healthc Eng. 2022. PMID: 35340250 Free PMC article.
-
Endostar Plus Apatinib Successfully Achieved Long Term Progression-Free Survival in Refractory Ovarian Cancer: A Case Report and Literature Review.Onco Targets Ther. 2021 Dec 1;14:5363-5372. doi: 10.2147/OTT.S335139. eCollection 2021. Onco Targets Ther. 2021. PMID: 34880628 Free PMC article.
-
Endostatin induces normalization of blood vessels in colorectal cancer and promotes infiltration of CD8+ T cells to improve anti-PD-L1 immunotherapy.Front Immunol. 2022 Oct 24;13:965492. doi: 10.3389/fimmu.2022.965492. eCollection 2022. Front Immunol. 2022. PMID: 36389685 Free PMC article.
-
Safety and efficacy of nivolumab plus recombinant human endostatin in previously treated advanced non-small-cell lung cancer.Transl Lung Cancer Res. 2022 Feb;11(2):201-212. doi: 10.21037/tlcr-22-49. Transl Lung Cancer Res. 2022. PMID: 35280309 Free PMC article.
-
Recombinant Endostatin as a Potential Radiosensitizer in the Treatment of Non-Small Cell Lung Cancer.Pharmaceuticals (Basel). 2023 Jan 31;16(2):219. doi: 10.3390/ph16020219. Pharmaceuticals (Basel). 2023. PMID: 37259367 Free PMC article. Review.
References
-
- International Agency for Research on Cancer. Cancer Today: Fact sheets. 2019. http://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf. Accessed 20 Mar, 2020.
-
- Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52(Pt 1):103–109. doi: 10.1016/j.semcancer.2017.11.019. - DOI - PMC - PubMed
-
- Pilkington G, Boland A, Brown T, Oyee J, Bagust A, Dickson R. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax. 2015;70(4):359–367. doi: 10.1136/thoraxjnl-2014-205914. - DOI - PubMed
-
- Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem and Biophys Res Commun. 2007;361(1):79–84. doi: 10.1016/j.bbrc.2007.06.155. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

