Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial
- PMID: 33087150
- PMCID: PMC7576974
- DOI: 10.1186/s12967-020-02573-9
Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial
Erratum in
-
Correction to: Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial.J Transl Med. 2021 Oct 21;19(1):442. doi: 10.1186/s12967-021-03094-9. J Transl Med. 2021. PMID: 34674735 Free PMC article. No abstract available.
Abstract
Background: Tocilizumab blocks pro-inflammatory activity of interleukin-6 (IL-6), involved in pathogenesis of pneumonia the most frequent cause of death in COVID-19 patients.
Methods: A multicenter, single-arm, hypothesis-driven trial was planned, according to a phase 2 design, to study the effect of tocilizumab on lethality rates at 14 and 30 days (co-primary endpoints, a priori expected rates being 20 and 35%, respectively). A further prospective cohort of patients, consecutively enrolled after the first cohort was accomplished, was used as a secondary validation dataset. The two cohorts were evaluated jointly in an exploratory multivariable logistic regression model to assess prognostic variables on survival.
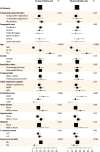
Results: In the primary intention-to-treat (ITT) phase 2 population, 180/301 (59.8%) subjects received tocilizumab, and 67 deaths were observed overall. Lethality rates were equal to 18.4% (97.5% CI: 13.6-24.0, P = 0.52) and 22.4% (97.5% CI: 17.2-28.3, P < 0.001) at 14 and 30 days, respectively. Lethality rates were lower in the validation dataset, that included 920 patients. No signal of specific drug toxicity was reported. In the exploratory multivariable logistic regression analysis, older age and lower PaO2/FiO2 ratio negatively affected survival, while the concurrent use of steroids was associated with greater survival. A statistically significant interaction was found between tocilizumab and respiratory support, suggesting that tocilizumab might be more effective in patients not requiring mechanical respiratory support at baseline.
Conclusions: Tocilizumab reduced lethality rate at 30 days compared with null hypothesis, without significant toxicity. Possibly, this effect could be limited to patients not requiring mechanical respiratory support at baseline. Registration EudraCT (2020-001110-38); clinicaltrials.gov (NCT04317092).
Keywords: COVID-19; Coronavirus; IL-6; Mortality; Phase 2; Pneumonia; Safety; Tocilizumab.
Conflict of interest statement
FP reports grants, personal fees and non-financial support from Bayer, personal fees from Sandoz, grants and personal fees from Incyte, personal fees from Celgene, grants and personal fees from Astra Zeneca, personal fees from Pierre Fabre, personal fees from Janssen Cilag, grants from Roche, grants from Pfizer, outside the submitted work.
MCP reports personal fees from Daichii Sankyo, personal fees from GSK, personal fees from MSD, grants from Roche, grants and personal fees from AstraZeneca, non-financial support from Bayer, outside the submitted work.
PAA reports grants and personal fees from BMS, grants and personal fees from Roche-Genentech, personal fees and other from MSD, grants and personal fees from Array, personal fees from Novartis, personal fees from Merck Serono, personal fees from Pierre Fabre, personal fees from Incyte, personal fees from Genmab, personal fees from NewLink Genetics, personal fees from Medimmune, personal fees from AstraZeneca, personal fees from Syndax, personal fees from Sun Pharma, personal fees from Sanofi, personal fees from Idera, personal fees from Ultimovacs, personal fees from Sandoz, personal fees from Immunocore, personal fees from 4SC, personal fees from Alkermes, personal fees from Italfarmaco, personal fees from Nektar, personal fees from Boehringer-Ingelheim, outside the submitted work.
CS reports grants and personal fees from Roche, personal fees from Sanofi-Genzyme, personal fees from Abbvie, personal fees from Pfizer, personal fees from Lilly, personal fees from Novartis, outside the submitted work.
FC reports grants from I am acting as Principal Investigator in company sponsored institutional clinical trials in the field of HIV (Gilead, ViiV, GSK, Janssen), HDV (Eiger) and coronavirus (Roche, Gilead), outside the submitted work.
AG reports non-financial support from Pfizer, outside the submitted work.
ML reports grants from Gilead, personal fees from Abbvie, personal fees from Merck, personal fees from Janseen, grants from Angelini, outside the submitted work.
The other Authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical