Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial
- PMID: 33087345
- PMCID: PMC7576328
- DOI: 10.1136/bmj.m3734
Regulatory T cells for minimising immune suppression in kidney transplantation: phase I/IIa clinical trial
Abstract
Objective: To assess whether reshaping of the immune balance by infusion of autologous natural regulatory T cells (nTregs) in patients after kidney transplantation is safe, feasible, and enables the tapering of lifelong high dose immunosuppression, with its limited efficacy, adverse effects, and high direct and indirect costs, along with addressing several key challenges of nTreg treatment, such as easy and robust manufacturing, danger of over immunosuppression, interaction with standard care drugs, and functional stability in an inflammatory environment in a useful proof-of-concept disease model.
Design: Investigator initiated, monocentre, nTreg dose escalation, phase I/IIa clinical trial (ONEnTreg13).
Setting: Charité-University Hospital, Berlin, Germany, within the ONE study consortium (funded by the European Union).
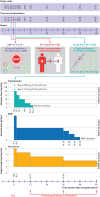
Participants: Recipients of living donor kidney transplant (ONEnTreg13, n=11) and corresponding reference group trial (ONErgt11-CHA, n=9).
Interventions: CD4+ CD25+ FoxP3+ nTreg products were given seven days after kidney transplantation as one intravenous dose of 0.5, 1.0, or 2.5-3.0×106 cells/kg body weight, with subsequent stepwise tapering of triple immunosuppression to low dose tacrolimus monotherapy until week 48.
Main outcome measures: The primary clinical and safety endpoints were assessed by a composite endpoint at week 60 with further three year follow-up. The assessment included incidence of biopsy confirmed acute rejection, assessment of nTreg infusion related adverse effects, and signs of over immunosuppression. Secondary endpoints addressed allograft functions. Accompanying research included a comprehensive exploratory biomarker portfolio.
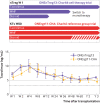
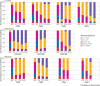
Results: For all patients, nTreg products with sufficient yield, purity, and functionality could be generated from 40-50 mL of peripheral blood taken two weeks before kidney transplantation. None of the three nTreg dose escalation groups had dose limiting toxicity. The nTreg and reference groups had 100% three year allograft survival and similar clinical and safety profiles. Stable monotherapy immunosuppression was achieved in eight of 11 (73%) patients receiving nTregs, while the reference group remained on standard dual or triple drug immunosuppression (P=0.002). Mechanistically, the activation of conventional T cells was reduced and nTregs shifted in vivo from a polyclonal to an oligoclonal T cell receptor repertoire.
Conclusions: The application of autologous nTregs was safe and feasible even in patients who had a kidney transplant and were immunosuppressed. These results warrant further evaluation of Treg efficacy and serve as the basis for the development of next generation nTreg approaches in transplantation and any immunopathologies.
Trial registration: NCT02371434 (ONEnTreg13) and EudraCT:2011-004301-24 (ONErgt11).
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the European Union 7th EU Framework Programme and Horizon 2020 programme and the BMBF under grant agreement BCRT and the BIH for the submitted work; no direct funding or donations from private parties, including the pharmaceutical industry; PR, HDV, and SLK also received unrestricted research funding from the public sources for the project (see funding).
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
