Eukaryotic Translation Elongation Factor 1 Delta Inhibits the Nuclear Import of the Nucleoprotein and PA-PB1 Heterodimer of Influenza A Virus
- PMID: 33087462
- PMCID: PMC7944447
- DOI: 10.1128/JVI.01391-20
Eukaryotic Translation Elongation Factor 1 Delta Inhibits the Nuclear Import of the Nucleoprotein and PA-PB1 Heterodimer of Influenza A Virus
Erratum in
-
Correction for Gao et al., "Eukaryotic Translation Elongation Factor 1 Delta Inhibits the Nuclear Import of the Nucleoprotein and PA-PB1 Heterodimer of Influenza A Virus".J Virol. 2021 Oct 13;95(21):e0141321. doi: 10.1128/JVI.01413-21. Epub 2021 Oct 13. J Virol. 2021. PMID: 34643117 Free PMC article. No abstract available.
Abstract
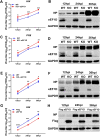
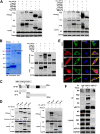
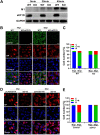
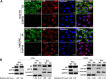
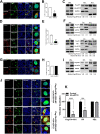
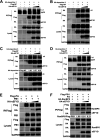
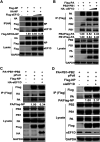
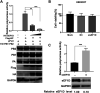
The viral ribonucleoprotein (vRNP) of the influenza A virus (IAV) is responsible for the viral RNA transcription and replication in the nucleus, and its functions rely on host factors. Previous studies have indicated that eukaryotic translation elongation factor 1 delta (eEF1D) may associate with RNP subunits, but its roles in IAV replication are unclear. Herein, we showed that eEF1D was an inhibitor of IAV replication because knockout of eEF1D resulted in a significant increase in virus yield. eEF1D interacted with RNP subunits polymerase acidic protein (PA), polymerase basic 1 (PB1), polymerase basic 2 (PB2), and also with nucleoprotein (NP) in an RNA-dependent manner. Further studies revealed that eEF1D impeded the nuclear import of NP and PA-PB1 heterodimer of IAV, thereby suppressing the vRNP assembly, viral polymerase activity, and viral RNA synthesis. Together, our studies demonstrate eEF1D negatively regulating the IAV replication by inhibition of the nuclear import of RNP subunits, which not only uncovers a novel role of eEF1D in IAV replication but also provides new insights into the mechanisms of nuclear import of vRNP proteins.IMPORTANCE Influenza A virus is the major cause of influenza, a respiratory disease in humans and animals. Different from most other RNA viruses, the transcription and replication of IAV occur in the cell nucleus. Therefore, the vRNPs must be imported into the nucleus for viral transcription and replication, which requires participation of host proteins. However, the mechanisms of the IAV-host interactions involved in nuclear import remain poorly understood. Here, we identified eEF1D as a novel inhibitor for the influenza virus life cycle. Importantly, eEF1D impaired the interaction between NP and importin α5 and the interaction between PB1 and RanBP5, which impeded the nuclear import of vRNP. Our studies not only reveal the molecular mechanisms of the nuclear import of IAV vRNP but also provide potential anti-influenza targets for antiviral development.
Keywords: NP; PA-PB1 heterodimer; eEF1D; influenza A virus; vRNP.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
KRT6A Restricts Influenza A Virus Replication by Inhibiting the Nuclear Import and Assembly of Viral Ribonucleoprotein Complex.Viruses. 2025 May 4;17(5):671. doi: 10.3390/v17050671. Viruses. 2025. PMID: 40431683 Free PMC article.
-
G Protein Subunit β1 Facilitates Influenza A Virus Replication by Promoting the Nuclear Import of PB2.J Virol. 2022 Jun 22;96(12):e0049422. doi: 10.1128/jvi.00494-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604143 Free PMC article.
-
Upregulation of galectin-3 in influenza A virus infection promotes viral RNA synthesis through its association with viral PA protein.J Biomed Sci. 2023 Feb 23;30(1):14. doi: 10.1186/s12929-023-00901-x. J Biomed Sci. 2023. PMID: 36823664 Free PMC article.
-
Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis.Nat Rev Microbiol. 2016 Aug;14(8):479-93. doi: 10.1038/nrmicro.2016.87. Epub 2016 Jul 11. Nat Rev Microbiol. 2016. PMID: 27396566 Free PMC article. Review.
-
Assembly and budding of influenza virus.Virus Res. 2004 Dec;106(2):147-65. doi: 10.1016/j.virusres.2004.08.012. Virus Res. 2004. PMID: 15567494 Free PMC article. Review.
Cited by
-
ARNT Inhibits H5N1 Influenza A Virus Replication by Interacting with the PA Protein.Viruses. 2022 Jun 21;14(7):1347. doi: 10.3390/v14071347. Viruses. 2022. PMID: 35891329 Free PMC article.
-
Avian Influenza Virus Tropism in Humans.Viruses. 2023 Mar 24;15(4):833. doi: 10.3390/v15040833. Viruses. 2023. PMID: 37112812 Free PMC article. Review.
-
Epstein-Barr virus immediate-early protein Zta mediates the proliferation and migration of HER2-overexpressing cancer cells.Arch Virol. 2023 May 3;168(5):150. doi: 10.1007/s00705-023-05774-x. Arch Virol. 2023. PMID: 37133552
-
Influenza Virus Host Restriction Factors: The ISGs and Non-ISGs.Pathogens. 2024 Jan 29;13(2):127. doi: 10.3390/pathogens13020127. Pathogens. 2024. PMID: 38392865 Free PMC article. Review.
-
KRT6A Restricts Influenza A Virus Replication by Inhibiting the Nuclear Import and Assembly of Viral Ribonucleoprotein Complex.Viruses. 2025 May 4;17(5):671. doi: 10.3390/v17050671. Viruses. 2025. PMID: 40431683 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

