CD8 T Cells Show Protection against Highly Pathogenic Simian Immunodeficiency Virus (SIV) after Vaccination with SIV Gene-Expressing BCG Prime and Vaccinia Virus/Sendai Virus Vector Boosts
- PMID: 33087465
- PMCID: PMC7851566
- DOI: 10.1128/JVI.01718-20
CD8 T Cells Show Protection against Highly Pathogenic Simian Immunodeficiency Virus (SIV) after Vaccination with SIV Gene-Expressing BCG Prime and Vaccinia Virus/Sendai Virus Vector Boosts
Abstract
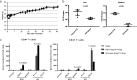
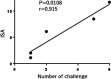
Toward development of a dual vaccine for human immunodeficiency virus type 1 (HIV-1) and tuberculosis infections, we developed a urease-deficient bacillus Calmette-Guérin (BCG) strain Tokyo172 (BCGΔurease) to enhance its immunogenicity. BCGΔurease expressing a simian immunodeficiency virus (SIV) Gag induced BCG antigen-specific CD4+ and CD8+ T cells more efficiently and more Gag-specific CD8+ T cells. We evaluated its protective efficacy against SIV infection in cynomolgus monkeys of Asian origin, shown to be as susceptible to infection with SIVmac251 as Indian rhesus macaques. Priming with recombinant BCG (rBCG) expressing SIV genes was followed by a boost with SIV gene-expressing LC16m8Δ vaccinia virus and a second boost with SIV Env-expressing Sendai virus. Eight weeks after the second boost, monkeys were repeatedly challenged with a low dose of SIVmac251 intrarectally. Two animals out of 6 vaccinees were protected, whereas all 7 control animals were infected without any early viral controls. In one vaccinated animal, which had the most potent CD8+ T cells in an in vitro suppression activity (ISA) assay of SIVmac239 replication, plasma viremia was undetectable throughout the follow-up period. Protection was confirmed by the lack of anamnestic antibody responses and detectable cell-associated provirus in various organs. Another monkey with a high ISA acquired a small amount of SIV, but it later became suppressed below the detection limit. Moreover, the ISA score correlated with SIV acquisition. On the other hand, any parameter relating anti-Env antibody was not correlated with the protection.IMPORTANCE Because both AIDS and tuberculosis are serious health threats in middle/low-income countries, development of a dual vaccine against them would be highly beneficial. To approach the goal, here we first assessed a urease-deficient bacillus Calmette-Guérin (BCG) for improvement of immunogenicity against both Mycobacterium tuberculosis and SIV. Second, we demonstrated the usefulness of Asian-origin cynomolgus monkeys for development of a preclinical AIDS vaccine by direct comparison with Indian rhesus macaques as the only validated hosts that identically mirror the outcomes of clinical trials, since the availability of Indian rhesus macaques is limited in countries other than the United States. Finally, we report the protective effect of a vaccination regimen comprising BCG, the highly attenuated vaccinia virus LC16m8Δ strain, and nontransmissible Sendai virus as safe vectors expressing SIV genes using repeated mucosal challenge with highly pathogenic SIVmac251. Identification of CD8+ T cells as a protective immunity suggests a future direction of AIDS vaccine development.
Keywords: CTL; HIV; SIV; human immunodeficiency virus; recombinant BCG; recombinant vaccinia; simian immunodeficiency virus.
Copyright © 2021 Kato et al.
Figures




References
-
- UNAIDS. 2019. UNAIDS Data 2019. UNAIDS, Geneva, Switzerland. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_....
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, HPTN 052 Study Team. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365:493–505. doi: 10.1056/NEJMoa1105243. - DOI - PMC - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. - DOI - PubMed
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JK. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

