Enterovirus Infection Induces Massive Recruitment of All Isoforms of Small Cellular Arf GTPases to the Replication Organelles
- PMID: 33087467
- PMCID: PMC7944459
- DOI: 10.1128/JVI.01629-20
Enterovirus Infection Induces Massive Recruitment of All Isoforms of Small Cellular Arf GTPases to the Replication Organelles
Abstract
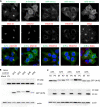
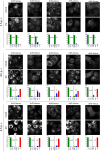
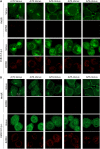
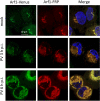
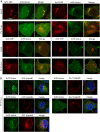
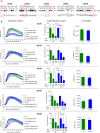
Enterovirus replication requires the cellular protein GBF1, a guanine nucleotide exchange factor for small Arf GTPases. When activated, Arfs associate with membranes, where they regulate numerous steps of membrane homeostasis. The requirement for GBF1 implies that Arfs are important for replication, but which of the different Arfs function(s) during replication remains poorly understood. Here, we established cell lines expressing each of the human Arfs fused to a fluorescent tag and investigated their behavior during enterovirus infection. Arf1 was the first to be recruited to the replication organelles, where it strongly colocalized with the viral antigen 2B and mature virions but not double-stranded RNA. By the end of the infectious cycle, Arf3, Arf4, Arf5, and Arf6 were also concentrated on the replication organelles. Once on the replication membranes, all Arfs except Arf3 were no longer sensitive to inhibition of GBF1, suggesting that in infected cells they do not actively cycle between GTP- and GDP-bound states. Only the depletion of Arf1, but not other class 1 and 2 Arfs, significantly increased the sensitivity of replication to GBF1 inhibition. Surprisingly, depletion of Arf6, a class 3 Arf, normally implicated in plasma membrane events, also increased the sensitivity to GBF1 inhibition. Together, our results suggest that GBF1-dependent Arf1 activation directly supports the development and/or functioning of the replication complexes and that Arf6 plays a previously unappreciated role in viral replication. Our data reveal a complex pattern of Arf activation in enterovirus-infected cells that may contribute to the resilience of viral replication in different cellular environments.IMPORTANCE Enteroviruses include many known and emerging pathogens, such as poliovirus, enteroviruses 71 and D68, and others. However, licensed vaccines are available only against poliovirus and enterovirus 71, and specific anti-enterovirus therapeutics are lacking. Enterovirus infection induces the massive remodeling of intracellular membranes and the development of specialized domains harboring viral replication complexes, replication organelles. Here, we investigated the roles of small Arf GTPases during enterovirus infection. Arfs control distinct steps in intracellular membrane traffic, and one of the Arf-activating proteins, GBF1, is a cellular factor required for enterovirus replication. We found that all Arfs expressed in human cells, including Arf6, normally associated with the plasma membrane, are recruited to the replication organelles and that Arf1 appears to be the most important Arf for enterovirus replication. These results document the rewiring of the cellular membrane pathways in infected cells and may provide new ways of controlling enterovirus infections.
Keywords: GBF1; GTPases Arf; RNA replication; enteroviruses; membrane metabolism; picornavirus; replication organelles.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
The development of resistance to an inhibitor of a cellular protein reveals a critical interaction between the enterovirus protein 2C and a small GTPase Arf1.PLoS Pathog. 2023 Sep 18;19(9):e1011673. doi: 10.1371/journal.ppat.1011673. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37721955 Free PMC article.
-
A Redundant Mechanism of Recruitment Underlies the Remarkable Plasticity of the Requirement of Poliovirus Replication for the Cellular ArfGEF GBF1.J Virol. 2019 Oct 15;93(21):e00856-19. doi: 10.1128/JVI.00856-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375590 Free PMC article.
-
Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1.J Virol. 2014 Mar;88(5):2725-36. doi: 10.1128/JVI.03650-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352456 Free PMC article.
-
Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review.
-
Allosteric regulation of Arf GTPases and their GEFs at the membrane interface.Small GTPases. 2016 Oct;7(4):283-296. doi: 10.1080/21541248.2016.1215778. Epub 2016 Jul 22. Small GTPases. 2016. PMID: 27449855 Free PMC article. Review.
Cited by
-
Picornaviruses: A View from 3A.Viruses. 2021 Mar 11;13(3):456. doi: 10.3390/v13030456. Viruses. 2021. PMID: 33799649 Free PMC article. Review.
-
Foot-and-Mouth Disease Virus 3A Hijacks Sar1 and Sec12 for ER Remodeling in a COPII-Independent Manner.Viruses. 2022 Apr 18;14(4):839. doi: 10.3390/v14040839. Viruses. 2022. PMID: 35458569 Free PMC article.
-
Mechanism, structural and functional insights into nidovirus-induced double-membrane vesicles.Front Immunol. 2024 Jun 11;15:1340332. doi: 10.3389/fimmu.2024.1340332. eCollection 2024. Front Immunol. 2024. PMID: 38919631 Free PMC article. Review.
-
Picornavirus translation strategies.FEBS Open Bio. 2022 Jun;12(6):1125-1141. doi: 10.1002/2211-5463.13400. Epub 2022 Mar 30. FEBS Open Bio. 2022. PMID: 35313388 Free PMC article. Review.
-
Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances.Pharmaceuticals (Basel). 2023 Jan 29;16(2):203. doi: 10.3390/ph16020203. Pharmaceuticals (Basel). 2023. PMID: 37259352 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

