Calmodulin inhibitors improve erythropoiesis in Diamond-Blackfan anemia
- PMID: 33087503
- PMCID: PMC7709865
- DOI: 10.1126/scitranslmed.abb5831
Calmodulin inhibitors improve erythropoiesis in Diamond-Blackfan anemia
Abstract
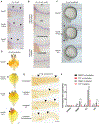
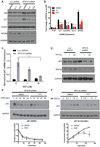
Diamond-Blackfan anemia (DBA) is a rare hematopoietic disease characterized by a block in red cell differentiation. Most DBA cases are caused by mutations in ribosomal proteins and characterized by higher than normal activity of the tumor suppressor p53. Higher p53 activity is thought to contribute to DBA phenotypes by inducing apoptosis during red blood cell differentiation. Currently, there are few therapies available for patients with DBA. We performed a chemical screen using zebrafish ribosomal small subunit protein 29 (rps29) mutant embryos that have a p53-dependent anemia and identified calmodulin inhibitors that rescued the phenotype. Our studies demonstrated that calmodulin inhibitors attenuated p53 protein amount and activity. Treatment with calmodulin inhibitors led to decreased p53 translation and accumulation but does not affect p53 stability. A U.S. Food and Drug Administration-approved calmodulin inhibitor, trifluoperazine, rescued hematopoietic phenotypes of DBA models in vivo in zebrafish and mouse models. In addition, trifluoperazine rescued these phenotypes in human CD34+ hematopoietic stem and progenitor cells. Erythroid differentiation was also improved in CD34+ cells isolated from a patient with DBA. This work uncovers a potential avenue of therapeutic development for patients with DBA.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


References
-
- Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, Meerpohl J, Karlsson S, Liu JM, Leblanc T, Paley C, Kang EM, Leder EJ, Atsidaftos E, Shimamura A, Bessler M, Glader B, Lipton JM, C. Participants of Sixth Annual Daniella Maria Arturi International Consensus, Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol 142, 859–876 (2008). - PMC - PubMed
-
- Draptchinskaia N, Gustavsson P, Andersson B, Pettersson M, Willig TN, Dianzani I, Ball S, Tchernia G, Klar J, Matsson H, Tentler D, Mohandas N, Carlsson B, Dahl N, The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat Genet 21, 169–175 (1999). - PubMed
-
- Ulirsch JC, Verboon JM, Kazerounian S, Guo MH, Yuan D, Ludwig LS, Handsaker RE, Abdulhay NJ, Fiorini C, Genovese G, Lim ET, Cheng A, Cummings BB, Chao KR, Beggs AH, Genetti CA, Sieff CA, Newburger PE, Niewiadomska E, Matysiak M, Vlachos A, Lipton JM, Atsidaftos E, Glader B, Narla A, Gleizes PE, O'Donohue MF, Montel-Lehry N, Amor DJ, McCarroll SA, O'Donnell-Luria AH, Gupta N, Gabriel SB, MacArthur DG, Lander ES, Lek M, Da Costa L, Nathan DG, Korostelev AA, Do R, Sankaran VG, Gazda HT, The Genetic Landscape of Diamond-Blackfan Anemia. Am J Hum Genet 103, 930–947 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R35 CA253125/CA/NCI NIH HHS/United States
- R24 OD017870/OD/NIH HHS/United States
- U01 HL134812/HL/NHLBI NIH HHS/United States
- R01 HL144780/HL/NHLBI NIH HHS/United States
- K01 DK121861/DK/NIDDK NIH HHS/United States
- U01 HL100001/HL/NHLBI NIH HHS/United States
- P01 HL032262/HL/NHLBI NIH HHS/United States
- P01 HL131477/HL/NHLBI NIH HHS/United States
- RC2 DK120535/DK/NIDDK NIH HHS/United States
- F32 HL124948/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R24 DK092760/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

