Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3
- PMID: 33087504
- PMCID: PMC7927160
- DOI: 10.1126/scitranslmed.abb7086
Toward allele-specific targeting therapy and pharmacodynamic marker for spinocerebellar ataxia type 3
Abstract
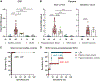
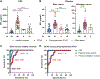
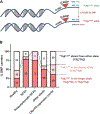
Spinocerebellar ataxia type 3 (SCA3), caused by a CAG repeat expansion in the ataxin-3 gene (ATXN3), is characterized by neuronal polyglutamine (polyQ) ATXN3 protein aggregates. Although there is no cure for SCA3, gene-silencing approaches to reduce toxic polyQ ATXN3 showed promise in preclinical models. However, a major limitation in translating putative treatments for this rare disease to the clinic is the lack of pharmacodynamic markers for use in clinical trials. Here, we developed an immunoassay that readily detects polyQ ATXN3 proteins in human biological fluids and discriminates patients with SCA3 from healthy controls and individuals with other ataxias. We show that polyQ ATXN3 serves as a marker of target engagement in human fibroblasts, which may bode well for its use in clinical trials. Last, we identified a single-nucleotide polymorphism that strongly associates with the expanded allele, thus providing an exciting drug target to abrogate detrimental events initiated by mutant ATXN3. Gene-silencing strategies for several repeat diseases are well under way, and our results are expected to improve clinical trial preparedness for SCA3 therapies.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

References
-
- Haberhausen G, Damian MS, Leweke F, Muller U, Spinocerebellar ataxia, type 3 (SCA3) is genetically identical to Machado–Joseph disease (MJD). J. Neurol. Sci 132, 71–75 (1995). - PubMed
-
- Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, Kimura J, Narumiya S, Kakizuka A, CAG expansions in a novel gene for Machado–Joseph disease at chromosome 14q32.1. Nat. Genet 8, 221–228 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

