IL-36R signaling integrates innate and adaptive immune-mediated protection against enteropathogenic bacteria
- PMID: 33087566
- PMCID: PMC7959549
- DOI: 10.1073/pnas.2004484117
IL-36R signaling integrates innate and adaptive immune-mediated protection against enteropathogenic bacteria
Abstract
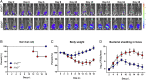
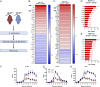
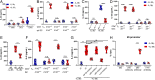
Enteropathogenic bacterial infections are a global health issue associated with high mortality, particularly in developing countries. Efficient host protection against enteropathogenic bacterial infection is characterized by coordinated responses between immune and nonimmune cells. In response to infection in mice, innate immune cells are activated to produce interleukin (IL)-23 and IL-22, which promote antimicrobial peptide (AMP) production and bacterial clearance. IL-36 cytokines are proinflammatory IL-1 superfamily members, yet their role in enteropathogenic bacterial infection remains poorly defined. Using the enteric mouse pathogen, C.rodentium, we demonstrate that signaling via IL-36 receptor (IL-36R) orchestrates a crucial innate-adaptive immune link to control bacterial infection. IL-36R-deficient mice (Il1rl2-/- ) exhibited significant impairment in expression of IL-22 and AMPs, increased intestinal damage, and failed to contain C. rodentium compared to controls. These defects were associated with failure to induce IL-23 and IL-6, two key IL-22 inducers in the early and late phases of infection, respectively. Treatment of Il1rl2-/- mice with IL-23 during the early phase of C. rodentium infection rescued IL-22 production from group 3 innate lymphoid cells (ILCs), whereas IL-6 administration during the late phase rescued IL-22-mediated production from CD4+ T cell, and both treatments protected Il1rl2-/- mice from uncontained infection. Furthermore, IL-36R-mediated IL-22 production by CD4+ T cells was dependent upon NFκB-p65 and IL-6 expression in dendritic cells (DCs), as well as aryl hydrocarbon receptor (AhR) expression by CD4+ T cells. Collectively, these data demonstrate that the IL-36 signaling pathway integrates innate and adaptive immunity leading to host defense against enteropathogenic bacterial infection.
Keywords: adaptive immunity; bacterial infection; innate immunity; interleukin.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

