Polymerase I and transcript release factor transgenic mice show impaired function of hematopoietic stem cells
- PMID: 33087586
- PMCID: PMC7655181
- DOI: 10.18632/aging.103729
Polymerase I and transcript release factor transgenic mice show impaired function of hematopoietic stem cells
Abstract
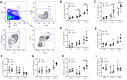
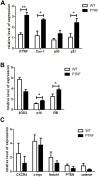
The age-dependent decline in stem cell function plays a critical role in aging, although the molecular mechanisms remain unclear. PTRF/Cavin-1 is an essential component in the biogenesis and function of caveolae, which regulates cell proliferation, endocytosis, signal transduction and senescence. This study aimed to analyze the role of PTRF in hematopoietic stem cells (HSCs) senescence using PTRF transgenic mice. Flow cytometry was used to detect the frequency of immune cells and hematopoietic stem/progenitor cells (HSCs and HPCs). The results showed than the HSC compartment was significantly expanded in the bone marrow of PTRF transgenic mice compared to age-matched wild-type (WT) mice, and exhibited the senescent phenotype characterized by G1 cell cycle arrest, increased SA-β-Gal activity and high levels of reactive oxygen species (ROS). The PTRF-overexpressing HSCs also showed significantly lower self-renewal and ability to reconstitute hematopoiesis in vitro and in vivo. Real-time PCR was performed to analyze the expression levels of senescence-related genes. PTRF induced HSCs senescence via the ROS-p38-p16 and caveolin-1-p53-p21 pathways. Furthermore, the PTRF+cav-1-/- mice showed similar HSCs function as WT mice, indicating that PTRF induces senescence in HSCs partly through caveolin-1. Thus PTRF impaired HSCs aging partly via caveolin-1.
Keywords: HSCs; PTRF; caveolin-1.
Conflict of interest statement
Figures




References
-
- Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA, Scharffetter-Kochanek K, Kestler HA, Zheng Y, Geiger H. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013; 503:392–96. 10.1038/nature12631 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

