Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia
- PMID: 33087718
- PMCID: PMC7578066
- DOI: 10.1038/s41467-020-19156-3
Dual mRNA therapy restores metabolic function in long-term studies in mice with propionic acidemia
Abstract
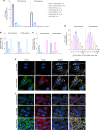
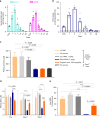
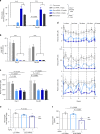
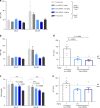
Propionic acidemia/aciduria (PA) is an ultra-rare, life-threatening, inherited metabolic disorder caused by deficiency of the mitochondrial enzyme, propionyl-CoA carboxylase (PCC) composed of six alpha (PCCA) and six beta (PCCB) subunits. We herein report an enzyme replacement approach to treat PA using a combination of two messenger RNAs (mRNAs) (dual mRNAs) encoding both human PCCA (hPCCA) and PCCB (hPCCB) encapsulated in biodegradable lipid nanoparticles (LNPs) to produce functional PCC enzyme in liver. In patient fibroblasts, dual mRNAs encoded proteins localize in mitochondria and produce higher PCC enzyme activity vs. single (PCCA or PCCB) mRNA alone. In a hypomorphic murine model of PA, dual mRNAs normalize ammonia similarly to carglumic acid, a drug approved in Europe for the treatment of hyperammonemia due to PA. Dual mRNAs additionally restore functional PCC enzyme in liver and thus reduce primary disease-associated toxins in a dose-dependent manner in long-term 3- and 6-month repeat-dose studies in PA mice. Dual mRNAs are well-tolerated in these studies with no adverse findings. These studies demonstrate the potential of mRNA technology to chronically administer multiple mRNAs to produce large complex enzymes, with applicability to other genetic disorders.
Conflict of interest statement
All authors are employees of, and receive salary and stock options from, Moderna, Inc.
Figures
Similar articles
-
Propionyl-CoA carboxylase pcca-1 and pccb-1 gene deletions in Caenorhabditis elegans globally impair mitochondrial energy metabolism.J Inherit Metab Dis. 2018 Mar;41(2):157-168. doi: 10.1007/s10545-017-0111-x. Epub 2017 Nov 20. J Inherit Metab Dis. 2018. PMID: 29159707 Free PMC article.
-
Translational Pharmacokinetic/Pharmacodynamic Model for mRNA-3927, an Investigational Therapeutic for the Treatment of Propionic Acidemia.Nucleic Acid Ther. 2023 Apr;33(2):141-147. doi: 10.1089/nat.2022.0036. Epub 2022 Dec 27. Nucleic Acid Ther. 2023. PMID: 36577040 Free PMC article.
-
Short-term rescue of neonatal lethality in a mouse model of propionic acidemia by gene therapy.Hum Gene Ther. 2009 Feb;20(2):169-80. doi: 10.1089/hum.2008.158. Hum Gene Ther. 2009. PMID: 19025475 Free PMC article.
-
Pathophysiological mechanisms of complications associated with propionic acidemia.Pharmacol Ther. 2023 Sep;249:108501. doi: 10.1016/j.pharmthera.2023.108501. Epub 2023 Jul 22. Pharmacol Ther. 2023. PMID: 37482098 Free PMC article. Review.
-
Propionyl-CoA carboxylase - A review.Mol Genet Metab. 2017 Dec;122(4):145-152. doi: 10.1016/j.ymgme.2017.10.002. Epub 2017 Oct 7. Mol Genet Metab. 2017. PMID: 29033250 Free PMC article. Review.
Cited by
-
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs.Nat Rev Mater. 2023;8(4):282-300. doi: 10.1038/s41578-022-00529-7. Epub 2023 Jan 19. Nat Rev Mater. 2023. PMID: 36691401 Free PMC article. Review.
-
Novel mRNA therapy restores GALT protein and enzyme activity in a zebrafish model of classic galactosemia.J Inherit Metab Dis. 2022 Jul;45(4):748-758. doi: 10.1002/jimd.12512. Epub 2022 May 27. J Inherit Metab Dis. 2022. PMID: 35527402 Free PMC article.
-
mRNA-based therapy proves superior to the standard of care for treating hereditary tyrosinemia 1 in a mouse model.Mol Ther Methods Clin Dev. 2022 Jul 15;26:294-308. doi: 10.1016/j.omtm.2022.07.006. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 35949297 Free PMC article.
-
A splice-switching oligonucleotide treatment ameliorates glycogen storage disease type 1a in mice with G6PC c.648G>T.J Clin Invest. 2023 Dec 1;133(23):e163464. doi: 10.1172/JCI163464. J Clin Invest. 2023. PMID: 37788110 Free PMC article.
-
Frameworks for transformational breakthroughs in RNA-based medicines.Nat Rev Drug Discov. 2024 Jun;23(6):421-444. doi: 10.1038/s41573-024-00943-2. Epub 2024 May 13. Nat Rev Drug Discov. 2024. PMID: 38740953 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

