Neonicotinoids disrupt circadian rhythms and sleep in honey bees
- PMID: 33087835
- PMCID: PMC7578099
- DOI: 10.1038/s41598-020-72041-3
Neonicotinoids disrupt circadian rhythms and sleep in honey bees
Abstract
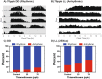
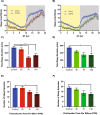
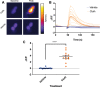
Honey bees are critical pollinators in ecosystems and agriculture, but their numbers have significantly declined. Declines in pollinator populations are thought to be due to multiple factors including habitat loss, climate change, increased vulnerability to disease and parasites, and pesticide use. Neonicotinoid pesticides are agonists of insect nicotinic cholinergic receptors, and sub-lethal exposures are linked to reduced honey bee hive survival. Honey bees are highly dependent on circadian clocks to regulate critical behaviors, such as foraging orientation and navigation, time-memory for food sources, sleep, and learning/memory processes. Because circadian clock neurons in insects receive light input through cholinergic signaling we tested for effects of neonicotinoids on honey bee circadian rhythms and sleep. Neonicotinoid ingestion by feeding over several days results in neonicotinoid accumulation in the bee brain, disrupts circadian rhythmicity in many individual bees, shifts the timing of behavioral circadian rhythms in bees that remain rhythmic, and impairs sleep. Neonicotinoids and light input act synergistically to disrupt bee circadian behavior, and neonicotinoids directly stimulate wake-promoting clock neurons in the fruit fly brain. Neonicotinoids disrupt honey bee circadian rhythms and sleep, likely by aberrant stimulation of clock neurons, to potentially impair honey bee navigation, time-memory, and social communication.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neonicotinoids disrupt memory, circadian behaviour and sleep.Sci Rep. 2021 Jan 21;11(1):2061. doi: 10.1038/s41598-021-81548-2. Sci Rep. 2021. PMID: 33479461 Free PMC article.
-
Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps.PLoS One. 2015 Aug 27;10(8):e0136928. doi: 10.1371/journal.pone.0136928. eCollection 2015. PLoS One. 2015. PMID: 26313444 Free PMC article.
-
A systematic review of honey bee (Apis mellifera, Linnaeus, 1758) infections and available treatment options.Vet Med Sci. 2023 Jul;9(4):1848-1860. doi: 10.1002/vms3.1194. Epub 2023 Jun 19. Vet Med Sci. 2023. PMID: 37335585 Free PMC article.
-
Moving past neonicotinoids and honeybees: A systematic review of existing research on other insecticides and bees.Environ Res. 2023 Oct 15;235:116612. doi: 10.1016/j.envres.2023.116612. Epub 2023 Jul 14. Environ Res. 2023. PMID: 37454798
-
The impact of landscape complexity and composition on honey bee visual learning.J Exp Biol. 2025 Jul 1;228(13):jeb250057. doi: 10.1242/jeb.250057. Epub 2025 Jul 4. J Exp Biol. 2025. PMID: 40554751 Free PMC article.
Cited by
-
Neonicotinoids disrupt memory, circadian behaviour and sleep.Sci Rep. 2021 Jan 21;11(1):2061. doi: 10.1038/s41598-021-81548-2. Sci Rep. 2021. PMID: 33479461 Free PMC article.
-
Methodology for Single Bee and Bee Brain 1H-NMR Metabolomics.Metabolites. 2021 Dec 13;11(12):864. doi: 10.3390/metabo11120864. Metabolites. 2021. PMID: 34940622 Free PMC article.
-
A neonicotinoid pesticide alters Drosophila olfactory processing.Sci Rep. 2023 Jun 30;13(1):10606. doi: 10.1038/s41598-023-37589-w. Sci Rep. 2023. PMID: 37391495 Free PMC article.
-
Effects of Thiamethoxam-Dressed Oilseed Rape Seeds and Nosema ceranae on Colonies of Apis mellifera iberiensis, L. under Field Conditions of Central Spain. Is Hormesis Playing a Role?Insects. 2022 Apr 9;13(4):371. doi: 10.3390/insects13040371. Insects. 2022. PMID: 35447813 Free PMC article.
-
Geographical and Seasonal Analysis of the Honeybee Microbiome.Microb Ecol. 2023 Feb;85(2):765-778. doi: 10.1007/s00248-022-01986-x. Epub 2022 Mar 14. Microb Ecol. 2023. PMID: 35284961 Free PMC article.
References
-
- Ferrier, P. M., Rucker, R. R., Thurman, W. N. & Burgett, M. Economic Effects and Responses to Changes in Honey Bee Health. www.ers.usda.gov (2018).
-
- Goulson D, Nicholls E, Botías C, Rotheray EL. Bee declines driven by combined Stress from parasites, pesticides, and lack of flowers. Science. 2015;347:1255957–1255957. - PubMed
-
- Tsvetkov N, et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science. 2017;356:1395–1397. - PubMed
-
- Woodcock BA, et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science. 2017;356:1393–1395. - PubMed
-
- Moore D. Honey bee circadian clocks: behavioral control from individual workers to whole-colony rhythms. J. Insect Physiol. 2001;47:843–857.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

