SPATA33 is an autophagy mediator for cargo selectivity in germline mitophagy
- PMID: 33087875
- PMCID: PMC7937689
- DOI: 10.1038/s41418-020-00638-2
SPATA33 is an autophagy mediator for cargo selectivity in germline mitophagy
Abstract
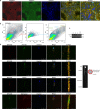
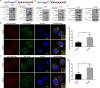
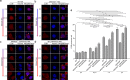
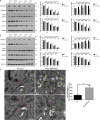
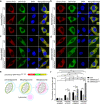
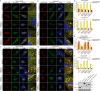
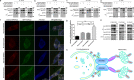
Selective autophagic degradation of mitochondria (mitophagy) is important in maintaining proper cellular homeostasis. Here, we found that SPATA33 is a novel autophagy mediator for mitophagy in testis. The SPATA33 protein localizes on mitochondria via its binding of the carboxyl terminal with the outer mitochondrial membrane protein VDAC2. Upon starvation induction, SPATA33 is recruited to autophagosome by binding the autophagy machinery ATG16L1 via its N-terminal along with mitochondria. Notably, Spata33 knockout inhibited autophagy and overexpression can promote autophagosome formation for mitochondrial sequestration. Therefore, SPATA33 confers selectivity for mitochondrial degradation and promotes mitophagy in male germline cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment in
-
SPATA33 functions as a mitophagy receptor in mammalian germline.Autophagy. 2021 May;17(5):1284-1286. doi: 10.1080/15548627.2021.1909836. Epub 2021 Apr 5. Autophagy. 2021. PMID: 33818286 Free PMC article.
Similar articles
-
SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. Proc Natl Acad Sci U S A. 2021. PMID: 34446558 Free PMC article.
-
SPATA33 functions as a mitophagy receptor in mammalian germline.Autophagy. 2021 May;17(5):1284-1286. doi: 10.1080/15548627.2021.1909836. Epub 2021 Apr 5. Autophagy. 2021. PMID: 33818286 Free PMC article.
-
BHRF1, a BCL2 viral homolog, disturbs mitochondrial dynamics and stimulates mitophagy to dampen type I IFN induction.Autophagy. 2021 Jun;17(6):1296-1315. doi: 10.1080/15548627.2020.1758416. Epub 2020 May 13. Autophagy. 2021. PMID: 32401605 Free PMC article.
-
Cargo recognition and degradation by selective autophagy.Nat Cell Biol. 2018 Mar;20(3):233-242. doi: 10.1038/s41556-018-0037-z. Epub 2018 Feb 23. Nat Cell Biol. 2018. PMID: 29476151 Free PMC article. Review.
-
Mechanisms and Physiological Roles of Mitophagy in Yeast.Mol Cells. 2018 Jan 31;41(1):35-44. doi: 10.14348/molcells.2018.2214. Epub 2018 Jan 23. Mol Cells. 2018. PMID: 29370687 Free PMC article. Review.
Cited by
-
SPATA33 affects the formation of cell adhesion complex by interacting with CTNNA3 in TM4 cells.Cell Tissue Res. 2022 Jul;389(1):145-157. doi: 10.1007/s00441-022-03631-y. Epub 2022 May 10. Cell Tissue Res. 2022. PMID: 35536443
-
SPATA33 localizes calcineurin to the mitochondria and regulates sperm motility in mice.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2106673118. doi: 10.1073/pnas.2106673118. Proc Natl Acad Sci U S A. 2021. PMID: 34446558 Free PMC article.
-
RAB37-mediated autophagy guards ovarian homeostasis and function.Autophagy. 2024 Dec;20(12):2738-2751. doi: 10.1080/15548627.2024.2389568. Epub 2024 Aug 29. Autophagy. 2024. PMID: 39113565 Free PMC article.
-
SPATA33 functions as a mitophagy receptor in mammalian germline.Autophagy. 2021 May;17(5):1284-1286. doi: 10.1080/15548627.2021.1909836. Epub 2021 Apr 5. Autophagy. 2021. PMID: 33818286 Free PMC article.
-
In situ architecture of the intercellular organelle reservoir between epididymal epithelial cells by volume electron microscopy.Nat Commun. 2025 Feb 15;16(1):1664. doi: 10.1038/s41467-025-56807-9. Nat Commun. 2025. PMID: 39955273 Free PMC article.
References
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

