Measuring clinical utility in the context of genetic testing: a scoping review
- PMID: 33087880
- PMCID: PMC7940410
- DOI: 10.1038/s41431-020-00744-2
Measuring clinical utility in the context of genetic testing: a scoping review
Abstract
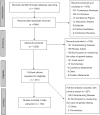
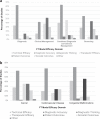
Standardized approaches to measuring clinical utility will enable more robust evaluations of genetic tests. To characterize how clinical utility has been measured, this scoping review examined outcomes used to operationalize this concept in the context of genetic testing, spanning relevant literature (2015-2017). The search strategy and analysis were guided by the Fryback and Thornbury hierarchical model of efficacy (FT Model). Through searches in Ovid MEDLINE, EMBASE and Web of Science, 194 publications were identified for inclusion. Two coders reviewed titles, abstracts, and full texts to determine eligibility. Results were analyzed using thematic and frequency analyses. This review generated a catalog of outcomes mapped to the efficacy domains of the FT Model. The degree of representation observed in each domain varied by the clinical purpose and clinical indication of genetic testing. Diagnostic accuracy (68%), technical (28.4%), and patient outcome (28.4%) efficacy studies were represented at the highest rate. Findings suggest that the FT Model is suitable for the genetics context however domain refinements may be warranted. More diverse clinical settings, robust study designs, and novel strategies for measuring clinical utility are needed.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Nelson B. Ensuring quality in genomic medicine: amid the rise in complex laboratory-developed tests, regulatory officials are seeking the right balance on quality assurance. Cancer Cytopathol. 2014;122:855–6. http://doi.wiley.com/10.1002/cncy.21499 - DOI - PubMed
-
- Sun F, Bruening W, Erinoff E, Schoelles KM. Addressing challenges in genetic test evaluation. addressing challenges genet test. Eval Eval Fram Assess Anal Validity. 2011. http://www.ncbi.nlm.nih.gov/pubmed/21834175 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

