TGF-β suppresses type 2 immunity to cancer
- PMID: 33087928
- PMCID: PMC8347705
- DOI: 10.1038/s41586-020-2836-1
TGF-β suppresses type 2 immunity to cancer
Abstract
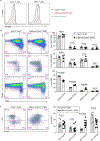
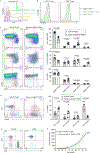
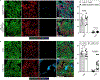
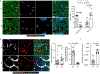
The immune system uses two distinct defence strategies against infections: microbe-directed pathogen destruction characterized by type 1 immunity1, and host-directed pathogen containment exemplified by type 2 immunity in induction of tissue repair2. Similar to infectious diseases, cancer progresses with self-propagating cancer cells inflicting host-tissue damage. The immunological mechanisms of cancer cell destruction are well defined3-5, but whether immune-mediated cancer cell containment can be induced remains poorly understood. Here we show that depletion of transforming growth factor-β receptor 2 (TGFBR2) in CD4+ T cells, but not CD8+ T cells, halts cancer progression as a result of tissue healing and remodelling of the blood vasculature, causing cancer cell hypoxia and death in distant avascular regions. Notably, the host-directed protective response is dependent on the T helper 2 cytokine interleukin-4 (IL-4), but not the T helper 1 cytokine interferon-γ (IFN-γ). Thus, type 2 immunity can be mobilized as an effective tissue-level defence mechanism against cancer.
Conflict of interest statement
Competing Interests
MSKCC has filed a patent application with the U.S. Patent and Trademark Office directed toward methods and compositions for targeting TGF-β signaling in CD4+ helper T cells for cancer immunotherapy.
Figures
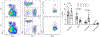






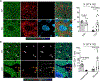



Comment in
-
Transforming growth factor β in breast cancer: another new trick for the old dog.Immunol Cell Biol. 2021 Mar;99(3):249-251. doi: 10.1111/imcb.12421. Epub 2020 Dec 6. Immunol Cell Biol. 2021. PMID: 33280167
-
Cancer Environment Immunotherapy: targeting TGF-β finds its way towards tissue healing and vasculature remodeling.Signal Transduct Target Ther. 2021 Jan 29;6(1):41. doi: 10.1038/s41392-021-00472-z. Signal Transduct Target Ther. 2021. PMID: 33514687 Free PMC article. No abstract available.
References
Methods References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

