Purification and biochemical characterization of an extracellular fructosyltransferase enzyme from Aspergillus niger sp. XOBP48: implication in fructooligosaccharide production
- PMID: 33088656
- PMCID: PMC7527383
- DOI: 10.1007/s13205-020-02440-w
Purification and biochemical characterization of an extracellular fructosyltransferase enzyme from Aspergillus niger sp. XOBP48: implication in fructooligosaccharide production
Abstract
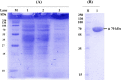
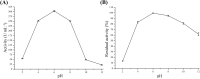
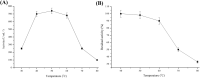
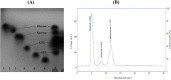
An extracellular fructosyltransferase (Ftase) enzyme with a molar mass of ≈70 kDa from a newly isolated indigenous coprophilous fungus Aspergillus niger sp. XOBP48 is purified to homogeneity and characterized in this study. The enzyme was purified to 4.66-fold with a total yield of 15.53% and specific activity of 1219.17 U mg-1 of protein after a three-step procedure involving (NH4)2SO4 fractionation, dialysis and anion exchange chromatography. Ftase showed optimum activity at pH 6.0 and temperature 50 °C. Ftase exhibited over 80% residual activity at pH range of 4.0-10.0 and ≈90% residual activity at temperature range of 40-60 °C for 6 h. Metal ion inhibitors Hg2+ and Ag+ significantly inhibited Ftase activity at 1 mmol concentration. Ftase showed K m, v max and k cat values of 79.51 mmol, 45.04 µmol min-1 and 31.5 min-1, respectively, with a catalytic efficiency (k cat/K m) of 396 µmol-1 min-1 for the substrate sucrose. HPLC-RI experiments identified the end products of fructosyltransferase activity as monomeric glucose, 1-kestose (GF2), and 1,1-kestotetraose (GF3). This study evaluates the feasibility of using this purified extracellular Ftase for the enzymatic synthesis of biofunctional fructooligosaccharides.
Keywords: 1,1-Kestotetraose; 1-Kestose; Aspergillus niger; Fructooligosaccharides; Fructosyltransferase; Zymography.
© King Abdulaziz City for Science and Technology 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures

References
-
- Adhikari P, et al. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella Enteritidis. Poult Sci. 2018;97(7):2525–2533. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. - PubMed
-
- Álvaro-Benito M, et al. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J Biotechnol. 2007;132:75–81. - PubMed
-
- Antosova M, Polakovic M. Fructosyltrasferase: the enzyme catalyzing production of fructooligosaccharides. Chem Pap. 2001;55(6):350–358.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

