Expression of huntingtin-associated protein 1 in adult mouse dorsal root ganglia and its neurochemical characterization in reference to sensory neuron subpopulations
- PMID: 33089002
- PMCID: PMC7560692
- DOI: 10.1016/j.ibror.2020.10.001
Expression of huntingtin-associated protein 1 in adult mouse dorsal root ganglia and its neurochemical characterization in reference to sensory neuron subpopulations
Abstract
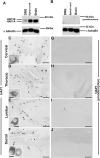
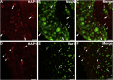
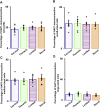
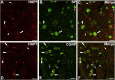
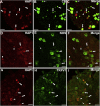
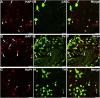
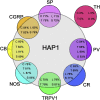
Huntingtin-associated protein 1 (HAP1) is a polyglutamine (polyQ) length-dependent interactor with causal agents in several neurodegenerative diseases and has been regarded as a protective factor against neurodegeneration. In normal rodent brain and spinal cord, HAP1 is abundantly expressed in the areas that are spared from neurodegeneration while those areas with little HAP1 are frequent targets of neurodegeneration. We have recently showed that HAP1 is highly expressed in the spinal dorsal horn and may participate in modification/protection of certain sensory functions. Neurons in the dorsal root ganglia (DRG) transmits sensory stimuli from periphery to spinal cord/brain stem. Nevertheless, to date HAP1 expression in DRG remains unreported. In this study, the expression of HAP1 in cervical, thoracic, lumbar and sacral DRG in adult male mice and its relationships with different chemical markers for sensory neurons were examined using Western blot and immunohistochemistry. HAP1-immunoreactivity was detected in the cytoplasm of DRG neurons, and the percentage of HAP1-immunoreactive (ir) DRG neurons was ranged between 28-31 %. HAP1-immunoreactivity was comparatively more in the small cells (47-58 %) and medium cells (40-44 %) than that in the large cells (9-11 %). Double-immunostaining for HAP1 and markers for nociceptive or mechanoreceptive neurons showed that about 70-80 % of CGRP-, SP-, CB-, NOS-, TRPV1-, CR- and PV-ir neurons expressed HAP1. In contrast, HAP1 was completely lacking in TH-ir neurons. Our current study is the first to clarify that HAP1 is highly expressed in nociceptive/proprioceptive neurons but absent in light-touch-sensitive TH neurons, suggesting the potential importance of HAP1 in pain transduction and proprioception.
Keywords: CB, calbindin; CGRP, calcitonin gene-related peptide; CR, calretinin; DAB, diaminobenzidine; DRG, dorsal root ganglia; HAP1, Huntingtin-associated protein 1; Huntingtin-associated protein 1; Iba1, ionized calcium-binding adapter molecule 1; Immunohistochemistry; LTMRs, low-threshold mechanoreceptors; MRGPR, Mas-related G-protein-coupled receptor; NDS, normal donkey serum; NOS, nitric oxide synthetase; NeuN, neuronal nuclei; Neurodegeneration; Neuroprotection; PB, phosphate buffer; PV, parvalbumin; Peripheral nervous system; SBMA, spinal and bulbar muscular atrophy; SP, substance P; STB, stigmoid body; Sensory neurons; TBST, Tris-buffered saline with 0.1 % Tween; TH, tyrosine hydroxylase; TRPV1, transient receptor potential vanilloid 1; VGLUT, vesicular glutamate transporter; htt, huntingtin; polyQ, polyglutamine.
© 2020 The Author(s).
Figures
Similar articles
-
Immunohistochemical expression and neurochemical phenotypes of huntingtin-associated protein 1 in the myenteric plexus of mouse gastrointestinal tract.Cell Tissue Res. 2021 Dec;386(3):533-558. doi: 10.1007/s00441-021-03542-4. Epub 2021 Oct 19. Cell Tissue Res. 2021. PMID: 34665322
-
Immunohistochemical analysis of huntingtin-associated protein 1 in adult rat spinal cord and its regional relationship with androgen receptor.Neuroscience. 2017 Jan 6;340:201-217. doi: 10.1016/j.neuroscience.2016.10.053. Epub 2016 Oct 29. Neuroscience. 2017. PMID: 27984179
-
Neurochemical phenotypes of huntingtin-associated protein 1 in reference to secretomotor and vasodilator neurons in the submucosal plexuses of rodent small intestine.Neurosci Res. 2023 Jun;191:13-27. doi: 10.1016/j.neures.2022.12.023. Epub 2022 Dec 26. Neurosci Res. 2023. PMID: 36581175
-
A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord.Brain Res Brain Res Rev. 1994 May;19(2):163-79. doi: 10.1016/0165-0173(94)90010-8. Brain Res Brain Res Rev. 1994. PMID: 8061685 Review.
-
Huntingtin-associated protein 1-associated intracellular trafficking in neurodegenerative diseases.Front Aging Neurosci. 2023 Feb 7;15:1100395. doi: 10.3389/fnagi.2023.1100395. eCollection 2023. Front Aging Neurosci. 2023. PMID: 36824265 Free PMC article. Review.
Cited by
-
Immunohistochemical expression and neurochemical phenotypes of huntingtin-associated protein 1 in the myenteric plexus of mouse gastrointestinal tract.Cell Tissue Res. 2021 Dec;386(3):533-558. doi: 10.1007/s00441-021-03542-4. Epub 2021 Oct 19. Cell Tissue Res. 2021. PMID: 34665322
-
Androgen Affects the Inhibitory Avoidance Memory by Primarily Acting on Androgen Receptor in the Brain in Adolescent Male Rats.Brain Sci. 2021 Feb 14;11(2):239. doi: 10.3390/brainsci11020239. Brain Sci. 2021. PMID: 33672867 Free PMC article.
-
Corneal Sensory Nerve Injury Disrupts Lacrimal Gland Function by Altering Circadian Rhythms in Mice.Invest Ophthalmol Vis Sci. 2025 Apr 1;66(4):40. doi: 10.1167/iovs.66.4.40. Invest Ophthalmol Vis Sci. 2025. PMID: 40238116 Free PMC article.
-
Immunohistochemical relationships of huntingtin-associated protein 1 with choline acetyltransferase in the forebrain cholinergic nuclei of adult mice.Cell Tissue Res. 2025 Jul 19. doi: 10.1007/s00441-025-03996-w. Online ahead of print. Cell Tissue Res. 2025. PMID: 40682600
-
Research advances in huntingtin-associated protein 1 and its application prospects in diseases.Front Neurosci. 2024 Jun 21;18:1402996. doi: 10.3389/fnins.2024.1402996. eCollection 2024. Front Neurosci. 2024. PMID: 38975245 Free PMC article. Review.
References
-
- Bartesaghi L., Wang Y., Fontanet P., Wanderoy S., Berger F., Wu H., Akkuratova N., Bouçanova F., Médard J.J., Petitpré C., Landy M.A., Zhang M.D., Harrer P., Stendel C., Stucka R., Dusl M., Kastriti M.E., Croci L., Lai H.C., Consalez G.G., Pattyn A., Ernfors P., Senderek J., Adameyko I., Lallemend F., Hadjab S., Chrast R. PRDM12 is required for initiation of the nociceptive neuron lineage during neurogenesis. Cell Rep. 2019;26:3484–3492. doi: 10.1016/j.celrep.2019.02.098. e4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

