Intranasal midazolam sedation as an effective sedation route in pediatric patients for radiologic imaging in the emergency ward: A single-blind randomized trial
- PMID: 33089024
- PMCID: PMC7549517
- DOI: 10.4103/2452-2473.297461
Intranasal midazolam sedation as an effective sedation route in pediatric patients for radiologic imaging in the emergency ward: A single-blind randomized trial
Abstract
Objectives: Prevention and reduction of pain, anxiety, and fear during medical procedures is one of the most important factors that should be considered in pediatric emergencies. The aim of this study was to compare the efficacy of oral versus intranasal midazolam in sedation during radiologic imaging in the largest province of Iran, Kerman.
Materials and methods: Eighty children were enrolled in this single-blind clinical trial based on convenience sampling and were divided into two groups receiving 0.5 mg/kg midazolam in oral route administration and 0.2 mg/kg midazolam in intranasal route administration. Finally, 75 patients remained for evaluating medication acceptability, sedation level, onset time of sedation, additional sedative dose, adverse effects of sedation, and provider satisfaction.
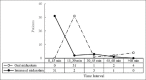
Results: Children in the intranasal group accepted medication more easily (89.8% vs. 36.9%; P ≤ 0.001), while these children received a lower sedation dose, but the sedation level in both methods was similar (P = 0.72). Our findings showed that children in the intranasal sedation group had a faster onset of sedation compared to the oral group (17.94 ± 8.99 vs. 34.50 ± 11.45; P ≤ 0.001). The frequency of midazolam side effects had no difference between the groups (29.7% vs. 15.8%; P = 0.15).
Conclusion: Intranasal midazolam with a lower sedation dose induces a faster onset and better acceptance. Intranasal midazolam can be used as an effective sedative method for pediatric patients, especially in emergency wards.
Keywords: Emergency ward; intranasal; midazolam; radiologic imaging.
Copyright: © 2020 Turkish Journal of Emergency Medicine.
Conflict of interest statement
Conflicts of interest None Declared.
Figures
Similar articles
-
Efficacy of intranasal sedation for pediatric dental procedures: a systematic review and meta-analysis.J Dent Anesth Pain Med. 2025 Feb;25(1):1-13. doi: 10.17245/jdapm.2025.25.1.1. Epub 2025 Jan 22. J Dent Anesth Pain Med. 2025. PMID: 39944848 Free PMC article. Review.
-
A comparison of the sedative effect of oral versus nasal midazolam combined with nitrous oxide in uncooperative children.Eur Arch Paediatr Dent. 2015 Oct;16(5):417-24. doi: 10.1007/s40368-015-0187-7. Epub 2015 May 5. Eur Arch Paediatr Dent. 2015. PMID: 25939638 Clinical Trial.
-
Comparison of the Sedative Effect of Inhaled Nitrous Oxide and Intranasal Midazolam in Behavior Management and Pain Perception of Pediatric Patients: A Split-mouth Randomized Controlled Clinical Trial.Int J Clin Pediatr Dent. 2021;14(Suppl 2):S111-S116. doi: 10.5005/jp-journals-10005-2085. Int J Clin Pediatr Dent. 2021. PMID: 35645472 Free PMC article.
-
Comparison of preanesthetic sedation in pediatric patients with oral and intranasal midazolam.J Anaesthesiol Clin Pharmacol. 2016 Jul-Sep;32(3):353-8. doi: 10.4103/0970-9185.168205. J Anaesthesiol Clin Pharmacol. 2016. PMID: 27625485 Free PMC article.
-
Comparing the Sedative Effect of Oral and Intranasal Midazolam and their Effect on Behavior in Pediatric Dental Patients.Int J Clin Pediatr Dent. 2022 Jan-Feb;15(1):128-134. doi: 10.5005/jp-journals-10005-2117. Int J Clin Pediatr Dent. 2022. PMID: 35528500 Free PMC article. Review.
Cited by
-
Efficacy of intranasal sedation for pediatric dental procedures: a systematic review and meta-analysis.J Dent Anesth Pain Med. 2025 Feb;25(1):1-13. doi: 10.17245/jdapm.2025.25.1.1. Epub 2025 Jan 22. J Dent Anesth Pain Med. 2025. PMID: 39944848 Free PMC article. Review.
-
Optimal Dose of Intranasal Midazolam for Procedural Sedation in Children: A Randomized Clinical Trial.JAMA Pediatr. 2025 Jul 28:e252181. doi: 10.1001/jamapediatrics.2025.2181. Online ahead of print. JAMA Pediatr. 2025. PMID: 40720114
-
Drugs for Procedural Sedation and Analgesia in Children: A Systematic Review and Meta-analysis.Drugs R D. 2025 Aug 13. doi: 10.1007/s40268-025-00522-9. Online ahead of print. Drugs R D. 2025. PMID: 40804602
-
Comparing the Sedative Effects of Intranasal Dexmedetomidine, Midazolam, and Ketamine in Outpatient Pediatric Surgeries: A Randomized Clinical Trial.Iran J Med Sci. 2024 Jul 1;49(7):421-429. doi: 10.30476/ijms.2023.99122.3118. eCollection 2024 Jul. Iran J Med Sci. 2024. PMID: 39114639 Free PMC article. Clinical Trial.
References
-
- Khoshrang H, Haddadi S, Farzi F, Ebrahimpour N. Comparing the effect of premedication with intra-nasal Dexmedetomidine and intra-nasal Midazolam on sedation and anxiety level in children undergoing elective surgery. J Anesthesiol Pain. 2016;6:1–10.
-
- Ayatollahi B, Behdad S. Evaluation of nasal Midazolam as a premedication anesthetic in preschool children. J Shahid Sadoughi Univ Med Sci. 2005;13:3–7.
-
- Hadley G, Maconochie I, Jackson A. A survey of intranasal medication use in the paediatric emergency setting in England and Wales. Emerg Med J. 2010;27:553–4. - PubMed
LinkOut - more resources
Full Text Sources