The G-protein-coupled chemoattractant receptor Fpr2 exacerbates neuroglial dysfunction and angiogenesis in diabetic retinopathy
- PMID: 33089077
- PMCID: PMC7566047
- DOI: 10.1096/fba.2020-00034
The G-protein-coupled chemoattractant receptor Fpr2 exacerbates neuroglial dysfunction and angiogenesis in diabetic retinopathy
Abstract
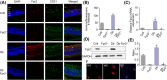
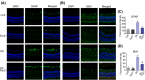
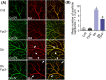
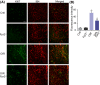
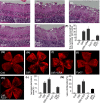
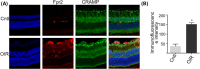
Diabetic retinopathy (DR) as a retinal neovascularization-related disease is one of the leading causes of irreversible blindness in patients. The goal of this study is to determine the role of a G-protein-coupled chemoattractant receptor (GPCR) FPR2 (mouse Fpr2) in the progression of DR, in order to identify novel therapeutic targets. We report that Fpr2 was markedly upregulated in mouse diabetic retinas, especially in retinal vascular endothelial cells, in associated with increased number of activated microglia and Müller glial cells. In contrast, in the retina of diabetic Fpr2 -/- mice, the activation of vascular endothelial cells and glia was attenuated with reduced production of proinflammatory cytokines. Fpr2 deficiency also prevented the formation of acellular capillary during diabetic progression. Furthermore, in oxygen-induced retinopathy (OIR) mice, the absence of Fpr2 was associated with diminished neovasculature formation and pathological vaso-obliteration region in the retina. These results highlight the importance of Fpr2 in exacerbating the progression of neuroglial degeneration and angiogenesis in DR and its potential as a therapeutic target.
Keywords: Fpr2; diabetic retinopathy; glial cell; neovascularization.
© 2020 The Authors. FASEB BioAdvances published by the Federation of American Societies for Experimental Biology.
Figures

References
-
- Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–277. - PubMed
-
- Stitt AW, Curtis TM, Chen M, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. - PubMed
-
- Rungger‐Brandle E, Dosso AA, Leuenberger PM. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41:1971–1980. - PubMed
LinkOut - more resources
Full Text Sources
