How Does Light Regulate Mood and Behavioral State?
- PMID: 33089172
- PMCID: PMC7445808
- DOI: 10.3390/clockssleep1030027
How Does Light Regulate Mood and Behavioral State?
Abstract
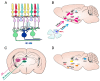
The idea that light affects mood and behavioral state is not new. However, not much is known about the particular mechanisms and circuits involved. To fully understand these, we need to know what properties of light are important for mediating changes in mood as well as what photoreceptors and pathways are responsible. Increasing evidence from both human and animal studies imply that a specialized class of retinal ganglion cells, intrinsically photosensitive retinal ganglion cells (ipRGCs), plays an important role in the light-regulated effects on mood and behavioral state, which is in line with their well-established roles in other non-visual responses (pupillary light reflex and circadian photoentrainment). This paper reviews our current understanding on the mechanisms and paths by which the light information modulates behavioral state and mood.
Keywords: alertness; behavioiral state; depression; ipRGCs; light; mood; non-visual responses.
© 2019 by the author.
Conflict of interest statement
Conflicts of InterestThe author declares no conflict of interest.
Figures

Similar articles
-
[Advances in researches of intrinsically photosensitive retinal ganglion cells].Zhonghua Yan Ke Za Zhi. 2020 Apr 11;56(4):308-312. doi: 10.3760/cma.j.cn112142-20190301-00098. Zhonghua Yan Ke Za Zhi. 2020. PMID: 32306623 Review. Chinese.
-
M1 ipRGCs Influence Visual Function through Retrograde Signaling in the Retina.J Neurosci. 2016 Jul 6;36(27):7184-97. doi: 10.1523/JNEUROSCI.3500-15.2016. J Neurosci. 2016. PMID: 27383593 Free PMC article.
-
Intrinsically photosensitive retinal ganglion cells.Rev Physiol Biochem Pharmacol. 2012;162:59-90. doi: 10.1007/112_2011_4. Rev Physiol Biochem Pharmacol. 2012. PMID: 22160822 Review.
-
Melanopsin-expressing intrinsically photosensitive retinal ganglion cells in retinal disease.Optom Vis Sci. 2014 Aug;91(8):894-903. doi: 10.1097/OPX.0000000000000284. Optom Vis Sci. 2014. PMID: 24879087 Review.
-
Synaptic influences on rat ganglion-cell photoreceptors.J Physiol. 2007 Jul 1;582(Pt 1):279-96. doi: 10.1113/jphysiol.2007.133751. Epub 2007 May 17. J Physiol. 2007. PMID: 17510182 Free PMC article.
Cited by
-
Comparative Effects of Red and Blue LED Light on Melatonin Levels During Three-Hour Exposure in Healthy Adults.Life (Basel). 2025 Apr 28;15(5):715. doi: 10.3390/life15050715. Life (Basel). 2025. PMID: 40430143 Free PMC article.
-
Emotion Detection Based on Pupil Variation.Healthcare (Basel). 2023 Jan 21;11(3):322. doi: 10.3390/healthcare11030322. Healthcare (Basel). 2023. PMID: 36766898 Free PMC article.
-
Multilevel and general linear modeling of weather and time effects on the emotional and behavioral states of children with profound intellectual and multiple disabilities.Front Psychiatry. 2024 Jan 5;14:1235582. doi: 10.3389/fpsyt.2023.1235582. eCollection 2023. Front Psychiatry. 2024. PMID: 38250279 Free PMC article.
-
Towards an evidence-based integrative lighting score: a proposed multi-level approach.Ann Med. 2024 Dec;56(1):2381220. doi: 10.1080/07853890.2024.2381220. Epub 2024 Jul 25. Ann Med. 2024. PMID: 39049780 Free PMC article. Review.
-
Designing artificial circadian environments with multisensory cares for supporting preterm infants' growth in NICUs.Front Neurosci. 2023 Aug 24;17:1152959. doi: 10.3389/fnins.2023.1152959. eCollection 2023. Front Neurosci. 2023. PMID: 37694118 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

