Modular timer networks: abdominal interneurons controlling the chirp and pulse pattern in a cricket calling song
- PMID: 33089402
- PMCID: PMC7603463
- DOI: 10.1007/s00359-020-01448-0
Modular timer networks: abdominal interneurons controlling the chirp and pulse pattern in a cricket calling song
Abstract
Chirping male crickets combine a 30 Hz pulse pattern with a 3 Hz chirp pattern to drive the rhythmic opening-closing movements of the front wings for sound production. Lesion experiments suggest two coupled modular timer-networks located along the chain of abdominal ganglia, a network in A3 and A4 generating the pulse pattern, and a network organized along with ganglia A4-A6 controlling the generation of the chirp rhythm. We analyzed neurons of the timer-networks and their synaptic connections by intracellular recordings and staining. We identified neurons spiking in phase with the chirps and pulses, or that are inhibited during the chirps. Neurons share a similar "gestalt", regarding the position of the cell body, the dendritic arborizations and the contralateral ascending axon. Activating neurons of the pulse-timer network elicits ongoing motor activity driving the generation of pulses; this activity is not structured in the chirp pattern. Activating neurons of the chirp-timer network excites pulse-timer neurons; it drives the generation of chirps and during the chirps the pulse pattern is produced. Our results support the hypothesis that two modular networks along the abdominal ganglion chain control the cricket calling song, a pattern generating network in the mesothoracic ganglion may not be required.
Keywords: Acoustic communication; Central pattern generator; Identified interneurons; Modular network; Timing of rhythms.
Conflict of interest statement
The authors declare no competing interest for this work.
Figures
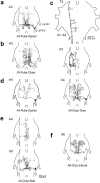



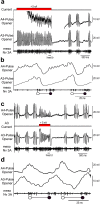

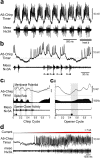
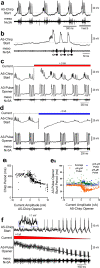
References
-
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by α1-adrenoceptors. Nature. 1985;315:501–503. - PubMed
-
- Alexander RD. Evolutionary change in cricket acoustical communication. Evolution. 1962;16(4):443–467.
-
- ASAB Ethics Committee Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav. 1997;53:229–234.
-
- Bentley DR. Intracellular activity in cricket neurons during generation of song patterns. Z vgl Physiol. 1969;283:267–283. - PubMed
-
- Bidaye SS, Bockemühl T, Büschges A. Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol. 2018;119:459–475. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

